Esophageal duplication cysts: a clinical practice review
Introduction
Esophageal duplication cysts are rare congenital anomalies that constitute between 0.5% and 2.5% of all esophageal masses (1). It is estimated that the incidence of esophageal duplication cysts is one in 8,200; they are twice as likely to occur in males when compared to females (2,3). The exact etiology of alimentary tract duplications is not well understood. However, it is thought to occur between the 4th and 8th weeks of development as a result of failure of intrauterine vacuolization of the esophagus (1). The duplication cysts lay within the esophageal wall, falling under the category of intramural lesions and need to be distinguished from esophageal malignancies, leiomyomas, lipomas, and gastrointestinal stromal tumors (GISTs). Esophageal duplications cyst can be lined by alimentary (squamous epithelium) or tracheobronchial mucosa regardless of where they are located. They have an envelope of smooth muscle and share a common wall with the esophagus (2,4). Up to one-third of esophageal duplication cysts contain heterotopic gastric mucosa; heterotopic pancreatic mucosa and mucosa consistent with Peyer’s patches have also been described (5,6). Approximately 80% of esophageal duplication cysts do not communicate with the esophageal lumen, whereas tubular duplications do have a direct communication with the lumen.
Clinical presentation
Patients with esophageal duplication cysts often remain asymptomatic, however those that become symptomatic usually present during childhood (1). During adulthood most esophageal duplication cysts are found incidentally while patients are undergoing work-up for unrelated conditions. Esophageal duplication cysts are most likely to be found in the lower third of the esophagus and the remaining are found in the upper/middle third of the esophagus (7). Clinical presentation varies depending on the location of the cyst. Those located within the upper esophagus are associated with respiratory manifestations including, dyspnea, stridor, respiratory distress, mass effect, retrosternal pain and cough. Whereas those located in the lower third of the esophagus are mainly associated with dysphagia, esophageal stenosis, food impaction and emesis. Although rare, hematemesis has been reported in the presence of heterotopic gastric mucosa. Additionally, cardiac arrhythmias, bleeding and rupture with complicated mediastinitis have also been reported in the literature (8). Due to the clockwise rotation of the stomach during embryogenesis, esophageal duplication cysts most commonly lay on the right lateral aspect of the esophagus (2).
Diagnosis
Benign esophageal lesions are often times asymptomatic and incidentally encountered during endoscopic or radiologic evaluation of the esophagus. Nevertheless, they pose challenges in establishing an accurate diagnosis and management plan. In the work-up of an esophageal duplication cyst, plain radiographs may demonstrate a well-defined soft tissue density in close association with the esophagus or widening of the mediastinum. A barium esophagram is an excellent study to delineate the anatomy of the esophagus, assess mucosal irregularities and visualize the location of the duplication cyst in relationship to surrounding organs. The esophagram can show an extrinsic versus and intramural compression on the wall of esophagus. Furthermore, intraluminal communication can also be recognized during this study. We routinely order an esophagram for pre-operative planning and post operatively to have a new baseline of the neo-anatomy post excision.
Computed tomography (CT), preferably, with intravenous contrast assists to delineate thick-walled cystic structures that can have complex versus simple fluid within. Generally, the cysts have smooth contour and low attenuation, with Hounsfield units ranging from 8 to 12 corresponding to water density. However, cysts can be filled with proteinaceous complex material, suffer spontaneous rupture or post instrumentation hemorrhage leading to images with hyper attenuating characteristics and elevated Hounsfield units closer to blood density (Figure 1). With the usage of oral contrast, filling defects are sometimes seen, but since duplication cysts are generally submucosal lesions, the lining appears smooth and with normal characteristics. While the differentiation between bronchogenic cysts and esophageal duplications cysts ca be challenging, a close relationship with the esophagus, lack of cartilaginous components and the presence of a double smooth muscle layer favors the diagnosis of an esophageal duplication cyst over bronchogenic cyst.
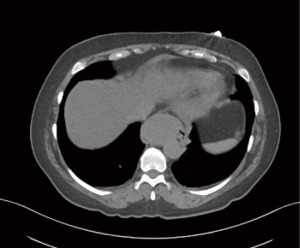
Further imaging modalities include magnetic resonance imaging (MRI) that shows the cystic nature of the mass as well as high intensity when imaged with T2-weighted sequences, regardless of the nature of the cyst contents. In the pediatric population, radionuclide scanning with Technetium 99m sodium pertechnetate assists with the localization of ectopic gastric mucosa, as the technetium is up taken by the gastric mucosa and this can be visualized with nuclear scans (9).
Positron emission tomography (PET) CT should only be ordered if malignant transformation of the duplication cyst has been confirmed with tissue biopsy. In this case, a PET scan will allow further investigation regarding invasion and whether there is spread to mediastinal or distant structures.
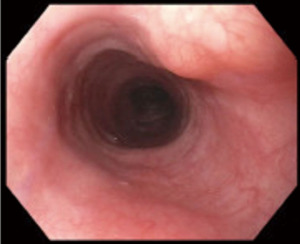
On esophagogastroscopy, duplication cysts appear as submucosal lesions with normal appearing esophageal mucosa (Figure 2), differential diagnosis include leiomyoma, GISTs and lipomas (10). Endoscopic ultrasound (EUS) demonstrates homogenous hypoechoic periesophageal multi-layered wall mass. Margins are generally smooth and the muscularis propria of the esophagus is in direct contact with the cyst wall (11).
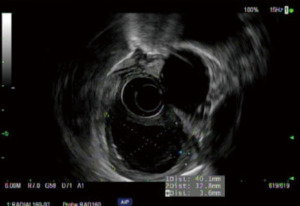
The role of EUS-guided fine needle aspiration (EUS-FNA) in the diagnosis of esophageal duplication cysts is controversial. Studies have shown an infection rate as high as 14% when EUS-FNA is performed (12,13). EUS-FNA should be reserved for lesions of indeterminate appearance, lesions concerning for malignancy and lesions that appear atypical (Figure 3).
Once diagnosis has been confirmed, an open discussion with patients regarding risks and benefits of surgical excision is recommended for a shared decision making approach. Surgery can be considered for both asymptomatic and symptomatic patients given the risk of future complications or malignant transformation (14,15). These lesions do not regress spontaneously and identification of the entire tract is key to ensure adequate resection. The surgical approach can be open, through a laparotomy or thoracotomy, or via minimally-invasive techniques dependent on the skill set of the surgeon. Minimally-invasive approaches have been demonstrated to be safe, feasible and with excellent outcomes (16,17). Given that the majority of esophageal duplication cysts are localized to the distal esophagus, herein we describe our trans-abdominal approach for surgical excision using the robotic platform. Cervical and thoracoscopic approaches are better reserved for upper and mid-esophageal lesions.
Surgical approach
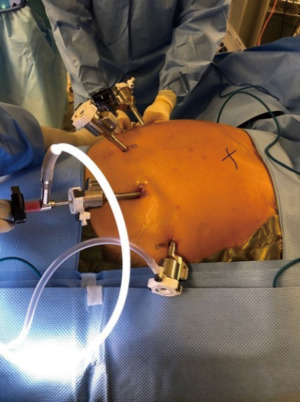
The patient is placed in the operating table with a footboard and all the pressure points adequately padded. Our patients receive preoperative antibiotics with a first generation cephalosporin and deep vein thrombosis prophylaxis routinely. A foley catheter and arterial line are also placed for continuous monitoring of the urine output and blood pressure. We utilize four robotic ports, the first (camera port) is placed 2 cm above and towards the left from the umbilicus. The second port is place in the left hemi abdomen, at the peritoneal reflexion, a third robotic port is placed in between the two prior ones and the fourth robotic arm is placed in the right upper quadrant. An assistant (12 mm) port goes in the left lower quadrant. Finally, a subxiphoid 5 mm port for the liver retractor (picture). the robot is routinely armed with the cadiere forceps (arm 1), camera (arm 2), vessel sealer (arm 3) and the curve bipolar and double fenestrated (interchange in arm 4) (Figure 4).
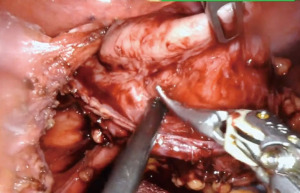
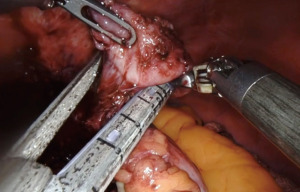
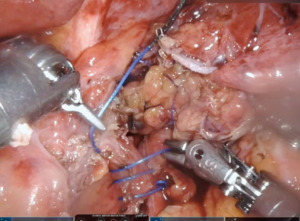
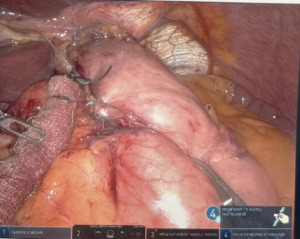
We start by dissecting at the phrenoesophageal membrane to enter the diaphanous plane of dissection. We are meticulous at visualizing and preserving both vagus nerves during the dissection. Thereafter, we dissect on the pars flacida towards the right crus preserving the peritoneal lining. We create a retro-esophageal window and utilize a Penrose drain for caudal and lateral traction. Finally, we mobilize the fundus by entering into the lesser sac. We take down the short gastric arteries with a combination of energy and hemostatic clips. We routinely extend our mediastinal circumferential esophageal dissection to the level of the inferior pulmonary veins, providing great exposure to the cystic mass. Thereafter, a myotomy is performed with blunt spreading and low energy. The myotomy is extended at least two centimeters proximally and distally to the cyst. Thereafter the cyst is enucleated with great care to avoid mucosal entry (Figures 5,6). Intraoperative evaluation with endoscopy and water and air tight test should be performed. A loose re-approximation of the myotomy is performed with 3.0 barbed absorbable sutures. A posterior cruroplasty with zero non absorbable barbed sutures is performed (Figure 7). If there is an associate large anterior defect, then an anterior cruroplasty is also performed. Finally, we perform a Dor fundoplication as it has been previously described (18) (Figure 8). If inadvertent mucosal entry were to happen and recognized intraoperatively, primary repair with 3.0 absorbable barbed sutures or interrupted PDS suture can be performed. We routinely perform an esophagram on post operative day #1, then begin patients on a liquid diet with discharge home within 48 hours of the index operation. Their diet is advanced at the follow-up appointment 1 week later.
Summary
Esophageal duplication cysts of the esophagus are infrequent and mostly asymptomatic. However, they can cause complications later in life and shared decision making with patients regarding surgical excision is indicated. Historically open posterolateral thoracotomy has been described as the traditional approach to mediastinal cyst. Despite the adequate visualization and reach that this approach provides, it has been associated with significant post-operative pain and prolonged length of stay in the hospital (19). More recently, a minimally invasive technique including thoracoscopic and laparoscopic approaches have been shown to be safe and successful. At our institution we prefer a robotic assisted approach. The robotic platform not only provides with a three-dimensional view and digital imaging, but also increases dexterity particularly while dissecting and suturing in a reduced space like the hiatus. Moreover, the robotic platform provides the operating surgeon with unparalleled autonomy. One of the greatest advantages that this platform provides is the capacity to control your own camera and obtain the best angle of visualization during the entire dissection. This translates in reduce risk of mucosal injury, improving surgical outcomes (20).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Nestor Villamizar) for the series “Mediastinal Cysts” published in Mediastinum. The article has undergone external peer review.
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-22-33/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-22-33/coif). The series “Mediastinal Cysts” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Whitaker JA, Deffenbaugh LD, Cooke AR. Esophageal duplication cyst. Case report. Am J Gastroenterol 1980;73:329-32. [PubMed]
- Arbona JL, Fazzi JG, Mayoral J. Congenital esophageal cysts: case report and review of literature. Am J Gastroenterol 1984;79:177-82. [PubMed]
- Anderson MC, Silberman WW, Shields TW. Duplications of the alimentary tract in the adult. Arch Surg 1962;85:94-108. [Crossref] [PubMed]
- Stern LE, Warner BW. Gastrointestinal duplications. Semin Pediatr Surg 2000;9:135-40. [Crossref] [PubMed]
- Ildstad ST, Tollerud DJ, Weiss RG, et al. Duplications of the alimentary tract. Clinical characteristics, preferred treatment, and associated malformations. Ann Surg 1988;208:184-9. [Crossref] [PubMed]
- Uppal P, Kaur J, Agarwala S, et al. Communicating oesophageal duplication cyst with heterotopic pancreatic tissue - an unusual cause of recurrent pneumonia in an infant. Acta Paediatr 2010;99:1432-3. [Crossref] [PubMed]
- Bhatia V, Tajika M, Rastogi A. Upper gastrointestinal submucosal lesions--clinical and endosonographic evaluation and management. Trop Gastroenterol 2010;31:5-29. [PubMed]
- Neo EL, Watson DI, Bessell JR. Acute ruptured esophageal duplication cyst. Dis Esophagus 2004;17:109-11. [Crossref] [PubMed]
- Jeung MY, Gasser B, Gangi A, et al. Imaging of cystic masses of the mediastinum. Radiographics 2002;22:S79-93. [Crossref] [PubMed]
- Liu R, Adler DG. Duplication cysts: Diagnosis, management, and the role of endoscopic ultrasound. Endosc Ultrasound 2014;3:152-60. [Crossref] [PubMed]
- Valli PV, Gubler C, Bauerfeind P. Severe Infectious Complications after Endoscopic Ultrasound-Guided Fine Needle Aspiration of Suspected Mediastinal Duplication Cysts: A Case Series. Inflamm Intest Dis 2017;1:165-71. [Crossref] [PubMed]
- Wildi SM, Hoda RS, Fickling W, et al. Diagnosis of benign cysts of the mediastinum: the role and risks of EUS and FNA. Gastrointest Endosc 2003;58:362-8. [Crossref] [PubMed]
- Cevasco M, Menard MT, Bafford R, et al. Acute infectious pseudoaneurysm of the descending thoracic aorta and review of infectious aortitis. Vasc Endovascular Surg 2010;44:697-700. [Crossref] [PubMed]
- Dai ZJ, Kang HF, Lin S, et al. Esophageal cancer with esophageal duplication cyst. Ann Thorac Surg 2013;96:e15-6. [Crossref] [PubMed]
- Sangüesa Nebot C, Llorens Salvador R, Carazo Palacios E, et al. Enteric duplication cysts in children: varied presentations, varied imaging findings. Insights Imaging 2018;9:1097-106. [Crossref] [PubMed]
- Surendran S, Samuel AS, Yacob M, et al. Minimally invasive surgery for adult oesophageal duplication cysts: Clinical profile and outcomes of treatment from a tertiary care centre and a review of literature. J Minim Access Surg 2021;17:525-31. [Crossref] [PubMed]
- Gonzalez-Urquijo M, Hinojosa-Gonzalez DE, Padilla-Armendariz DP, et al. Esophageal Duplication Cysts in 97 Adult Patients: A Systematic Review. World J Surg 2022;46:154-62. [Crossref] [PubMed]
- Horgan S, Galvani C, Gorodner MV, et al. Robotic-assisted Heller myotomy versus laparoscopic Heller myotomy for the treatment of esophageal achalasia: multicenter study. J Gastrointest Surg 2005;9:1020-9; discussion 1029-30. [Crossref] [PubMed]
- Herbella FA, Tedesco P, Muthusamy R, et al. Thoracoscopic resection of esophageal duplication cysts. Dis Esophagus 2006;19:132-4. [Crossref] [PubMed]
- Obasi PC, Hebra A, Varela JC. Excision of esophageal duplication cysts with robotic-assisted thoracoscopic surgery. JSLS 2011;15:244-7. [Crossref] [PubMed]
Cite this article as: Wahi JE, Safdie FM. Esophageal duplication cysts: a clinical practice review. Mediastinum 2023;7:1.