
Good’s syndrome and COVID-19: case report and literature review
Introduction
Good’s syndrome (GS) is a rare adult-onset acquired immunodeficiency that involves thymoma, hypogammaglobulinemia (HGG), and a significantly reduced or absence of peripheral B cells (1). The incidence rate of GS is estimated to be 1.5 per 1,000,000. GS was first described in 1954 by Robert Good as a rare association between thymoma, invasive bacterial infections, and HGG, often occurring between the fourth and sixth decades of life (2). Despite the fact that GS has been known for over sixty years, the exact pathophysiology is still unclear. The majority of data on GS is from case reports or small case series, making it difficult to investigate the underlying pathogenesis of the disease. Nevertheless, research in recent years have elucidated several aspects of GS and have clarified some previously held misunderstandings regarding its immunopathology. Initially, researchers suggested that GS was a subset of common variable immunodeficiency (CVID) due to similar patterns of occurrence of invasive bacterial and opportunistic infections. Despite similarities in clinical presentation, the underlying genetic backgrounds and immune pathologies differ (3). Studies have found no conclusive evidence for disease-causing genetic variants associated with GS. Until now, only three genetic studies have been conducted, including a total of eight patients. Two of these patients were reported to have a mutation in transmembrane activator and CAML interactor (TACI), and one patient had two missense mutations in B-cell activating factor receptor (BAFF-R). Both of these genes are members of the tumour necrosis factor receptor (TNFR) superfamily and are involved with the maturation and homeostasis of B cells. In CVID, monogenic defects are present in some patients, and variants of the previously mentioned genes have also been associated with CVID (4-6). The majority of CVID patients have normal to moderate levels of B cells, but with defects in peripheral B cell differentiation, survival and antibody production. In GS, patients generally lack B cells, which is more similar to patients with agammaglobulinemia, and suggests a defect in lymphopoiesis during the early B cell development (2).
The disease presentation of GS is variable and recognition of GS across a range of clinical manifestations is challenging, often leading to diagnostic delay. Recurrent bacterial infections, increased rates of opportunistic infections such as Pneumocystis jirovecii, mucocutaneous candidiasis, and reactivation of latent viruses such as herpes simplex virus and varicella zoster are one of the main clinical features of GS (7). Thymoma is also one of the main clinical features of GS, and is often found incidentally during workup for a suspected myasthenia gravis (MG) or in patients investigated for recurrent pulmonary infections. The majority of thymomas in GS are benign and localized. Thymectomy has not been shown to improve HGG in patients with GS, indicating that thymoma management alone is not sufficient as treatment (2). Treatment for GS is mainly supportive and includes antimicrobials and immunoglobulin replacement. Infections remain one of the leading causes of mortality in GS and the prognosis is believed to be worse compared to other adult immunodeficiencies (8). In the last two years, coronavirus disease 2019 (COVID-19) has shown to have higher morbidity and mortality rates in patients with primary and secondary immunodeficiencies compared to the general population (1). However, there is a paucity of data regarding the susceptibility to COVID-19 patients and the clinical outcomes of COVID-19 in patients with GS. In this case report, we describe a patient who presented with GS and a simultaneous COVID-19 infection. Details of the case and a review of the latest data on GS are presented. We present the following case in accordance with the CARE reporting checklist (available at https://med.amegroups.com/article/view/10.21037/med-22-12/rc).
Case presentation
A 53-year-old man presented to the department of dermatology with symptoms of oral lichen planus and a papular erythematous rash on chest and arms. The patient had previously been treated with 2 courses of methylprednisolone at a maximum of 32 mg/day, which was slowly tapered to a maintenance dose of 2 mg every 2 days. However, the patient’s symptoms recurred both times after lowering the dose below 4 mg/day. Additional symptoms included a weight loss of 5 kilograms in the last six months. The patient had no relevant medical or family history. Initially, the patient had been treated with corticosteroids and hydroxychloroquine. However, the patient noticed that the symptoms returned after several attempts to stop with the initial corticosteroids treatment. For this reason, the treatment regimen was changed to acitretin, a second generation retinoid.
Work-up with laboratory tests and a chest X-ray were performed, with the latter showing evidence of lymphadenopathy (not shown). Subsequent computed tomography (CT) and positron emission tomography (PET) of the chest showed a large anterior mediastinal mass with a maximum diameter of 10 centimetres (Figure 1A,1B and Figure 2). A core needle biopsy was performed, which showed spindle-shaped cells and lymphoid cells. Additional tests showed terminal deoxynucleotidyl transferase (TdT), cluster of differentiation 3 (CD3), and CD5 positivity in the lymphoid cells. Furthermore, pancytokeratin (CKpan) was positive in the spindle-shaped cells. This led to a pathological diagnosis of a thymoma type AB. Furthermore, flow cytometry showed a low B-cell count and CD4+/CD8+ ratio (Table 1). Acetylcholine receptor antibodies were not present.
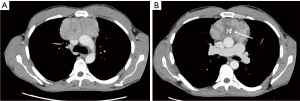
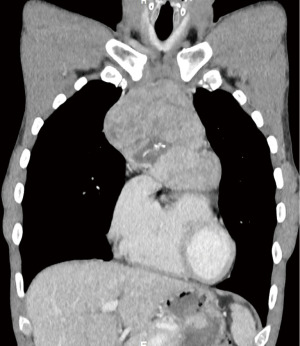
Table 1
| Cell population | Value | Normal range | Unit |
|---|---|---|---|
| T cell: CD3 | 778 | 700–2,100 | cells/µL |
| T helper cells: CD4+/CD3+ | 240 | 300–1,400 | cells/µL |
| CD4+/CD8+ ratio | 0.49 | 1–3.6 | – |
| B cell: CD19 | <6 | 100–500 | cells/µL |
CD, cluster of differentiation.
In addition to these findings, laboratory analysis revealed HGG (Table 2). The combination of a thymoma and HGG led to a diagnosis of GS for which treatment with immunoglobulin therapy was initiated at a dose of 25 grams every 4 weeks. Due to the volume of the mass and the close relation with major cardiac and pulmonary vessels, a multidisciplinary decision was made to administer induction chemotherapy with cisplatin-etoposide prior to surgery. After 2 cycles, the patient developed a cough, loss of taste and smell, dyspnoea, and fatigue. He was admitted to the hospital and was diagnosed with COVID-19. Initially, the patient was subfebrile, however, he developed a fever (39.8 °C) within 4 days. Oxygen therapy at 2 L/min was initiated due to low blood oxygen saturations. The patient was discharged in a stable condition after two weeks.
Table 2
| Immunoglobulin | Value (g/L) | Normal range (g/L) |
|---|---|---|
| IgG (turbidimetric) | 2.45 | 6.5–16 |
| IgA (turbidimetric) | <0.15 | 0.4–3.5 |
| IgA (nephelometric) | 0.12 | 0.7–4.0 |
IgG, immunoglobulin G; IgA, Immunoglobulin A.
Six weeks after the initial PET-CT, a new chest CT-scan showed no evidence of volume reduction. Despite the fact that there was no volume reduction, a multidisciplinary decision was made to perform a surgical resection of the mass via sternotomy. However, screening for COVID-19 at time of admission was positive, despite absence of any symptoms. Due to the patient’s prolonged COVD-19 viral shedding, the immunocompromised state, and the absence of COVID-19 seroconversion (no COVID-19 immunoglobulins on serology), treatment with remdesivir, a broad-spectrum antiviral therapy, was initiated according to the national guidelines at that time. After one week of treatment, a thymectomy via sternotomy was performed. Final pathology confirmed a thymoma type AB, fully encapsulated, without signs of invasion, and with a maximum diameter of 14 centimetres. Resection margins were negative and the tumour was classified as pT1aR0.
Due to persistent COVID-19 shedding in the postoperative period, convalescent plasma was administered. However, this did not have any effect on the asymptomatic COVID-19 shedding. The patient was discharged in excellent condition. The patient received two doses of an mRNA vaccination 14 weeks after his discharge. However, even after vaccination, the COVID-19 shedding continued, which indicates that the patient is a vaccine non-responder. Since then, the patient has received immunoglobulin treatments at a dose of 350–400 mg/kg every 4 weeks for his GS and has not developed any new infections since the start of this therapy. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Current evidence suggests that GS is a secondary immunodeficiency that is induced by thymic tumours. In a systematic review by Kelesidis et al., invasive spindle cell thymomas were the most common type associated with GS (9). However, in a retrospective survey by Zaman et al., 59% of patients with GS had AB as histological type of tumour (10). Current data suggests that the time of diagnosis of the thymoma often differs between patients; the thymoma diagnosis can precede, occur concurrently, or follow the diagnosis of HGG (2). In the study by Zaman et al., the median age of GS diagnosis was 58 years (range, 51–62 years) with a median interval of four years between the diagnosis of the thymoma and HGG. Previous studies have not shown any significant effects of thymectomy on the immunological symptoms of these patients (2,9). This has led some authors to believe that thymomas are more likely to be a clinical result than the driving factor behind the B cell depletion in GS patients. Nevertheless, a number of studies have shown that the thymic tumour microenvironment can cause an aberrant maturation of T cell precursors, leading to an altered T cell subset in the blood. However, the majority of data is derived from studies regarding MG (11-13).
No genetic defects in B cell differentiation have yet been identified in patients with GS, which suggests that there may be some intrinsic and/or extrinsic factors driving the B cell lymphopenia. In a study by Masci et al., an oligoclonal expansion of a subset of CD8 T cells with a vβ8 T cell receptor was found in the bone marrow of patients with thymoma and B cell lymphopenia. However, this subset expansion was not demonstrated in the same patients’ peripheral blood lymphocyte population. Further analyses using genetic sequencing revealed that this could be an antigen-specific response to a previously unknown pathogen or an autoimmune reaction towards B cell progenitors (14). Some authors have hypothesized that the development of the autoimmune response in GS patients could be multi-factorial as well. A number of viruses and bacteria have been postulated to cause auto-immunity through epitope spreading, molecular mimicry, and cross-reactive antibody production. No pathogenic agent has yet been identified (15).
Regarding the possible association between a COVID-19 infection and GS, only a few cases have been published. There is controversy regarding the way in which patients with antibody deficiencies react to COVID-19. Some authors have even suggested that the intrinsic lack of B cells could be considered an advantage in COVID-19 by preventing the development of a hyper inflammation state (16). Several case studies of patients with agammaglobulinemia showed that, paradoxically, patients with B cell deficiencies showed milder courses of disease and did not require mechanical ventilation (17-19). In our patient, the COVID-19 infection was not severe and conservative measures proved to be sufficient. Nevertheless, our patient did demonstrate prolonged COVID-19 shedding 14 weeks postoperatively, even after two doses of mRNA vaccinations. Regular administration of immunoglobulin replacement therapy has resulted in a stable condition without any new signs of infection. More data regarding the aetiology and underlying immunopathology are necessary to fully understand GS and to create better treatment options. Furthermore, more data on the clinical outcomes of COVID-19 infections in GS patients is needed to see whether this patient population is more prone to develop severe outcomes than the general population.
Patient perspective
Our patient mentioned that the first weeks after his diagnosis were quite stressful, mainly due to the uncertainty regarding his diagnosis and the treatment plan. The numerous visits to the hospital put quite a lot of strain on our patient, keeping in mind that he had no relevant medical history prior to his new diagnosis of GS. The fear of the effects of the COVID-19 infection further increased the patients worries regarding his health. However, following the successful operation and improvement of his clinical symptoms, our patient reported to be less stressed about his (future) health status.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://med.amegroups.com/article/view/10.21037/med-22-12/rc
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-22-12/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-22-12/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lindahl H, Smith CIE, Bergman P. COVID-19 in a patient with Good's syndrome and in 13 patients with common variable immunodeficiency. Clinical Immunology Communications 2021;1:20-4. [Crossref]
- Kabir A, Alizadehfar R, Tsoukas CM. Good's Syndrome: Time to Move on From Reviewing the Past. Front Immunol 2022;12:815710. [Crossref] [PubMed]
- Guevara-Hoyer K, Fuentes-Antrás J, Calatayud Gastardi J, et al. Immunodeficiency and thymoma in Good syndrome: Two sides of the same coin. Immunol Lett 2021;231:11-7. [Crossref] [PubMed]
- Margraf RL, Coonrod EM, Durtschi JD, et al. TACI mutation p.Lys154Ter identified in Good Syndrome. Clin Immunol 2013;146:10-2. [Crossref] [PubMed]
- Sáenz-Cuesta M, Martínez-Pomar N, de Gracia J, et al. TACI mutation in Good's Syndrome: in search of a genetic basis. Clin Immunol 2012;145:27-30. [Crossref] [PubMed]
- Salzer U, Bacchelli C, Buckridge S, et al. Relevance of biallelic versus monoallelic TNFRSF13B mutations in distinguishing disease-causing from risk-increasing TNFRSF13B variants in antibody deficiency syndromes. Blood 2009;113:1967-76. [Crossref] [PubMed]
- Tarr PE, Sneller MC, Mechanic LJ, et al. Infections in patients with immunodeficiency with thymoma (Good syndrome). Report of 5 cases and review of the literature. Medicine (Baltimore) 2001;80:123-33. [Crossref] [PubMed]
- Shi Y, Wang C. When the Good Syndrome Goes Bad: A Systematic Literature Review. Front Immunol 2021;12:679556. [Crossref] [PubMed]
- Kelesidis T, Yang O. Good's syndrome remains a mystery after 55 years: A systematic review of the scientific evidence. Clin Immunol 2010;135:347-63. [Crossref] [PubMed]
- Zaman M, Huissoon A, Buckland M, et al. Clinical and laboratory features of seventy-eight UK patients with Good's syndrome (thymoma and hypogammaglobulinaemia). Clin Exp Immunol 2019;195:132-8. [Crossref] [PubMed]
- Kelleher P, Misbah SA. What is Good's syndrome? Immunological abnormalities in patients with thymoma. J Clin Pathol 2003;56:12-6. [Crossref] [PubMed]
- Weksler B, Lu B. Alterations of the immune system in thymic malignancies. J Thorac Oncol 2014;9:S137-42. [Crossref] [PubMed]
- Yamada Y, Weis CA, Thelen J, et al. Thymoma Associated Myasthenia Gravis (TAMG): Differential Expression of Functional Pathways in Relation to MG Status in Different Thymoma Histotypes. Front Immunol 2020;11:664. [Crossref] [PubMed]
- Masci AM, Palmieri G, Vitiello L, et al. Clonal expansion of CD8+ BV8 T lymphocytes in bone marrow characterizes thymoma-associated B lymphopenia. Blood 2003;101:3106-8. [Crossref] [PubMed]
- Smatti MK, Cyprian FS, Nasrallah GK, et al. Viruses and Autoimmunity: A Review on the Potential Interaction and Molecular Mechanisms. Viruses 2019;11:762. [Crossref] [PubMed]
- Duarte M, Faria L, Patronillo C, et al. A Case of Severe COVID-19 in a Patient with Good’s Syndrome. Eur J Case Rep Intern Med 2021;8:002976. [Crossref] [PubMed]
- Babaha F, Rezaei N. Primary Immunodeficiency Diseases in COVID-19 Pandemic: A Predisposing or Protective Factor? Am J Med Sci 2020;360:740-1. [Crossref] [PubMed]
- Soresina A, Moratto D, Chiarini M, et al. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr Allergy Immunol 2020;31:565-9. [Crossref] [PubMed]
- Quinti I, Lougaris V, Milito C, et al. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J Allergy Clin Immunol 2020;146:211-213.e4. [Crossref] [PubMed]
Cite this article as: Berzenji L, Yogeswaran SK, Snoeckx A, Van Schil PE, Wener R, Hendriks JMH. Good’s syndrome and COVID-19: case report and literature review. Mediastinum 2023;7:5.


