
The therapeutic relevance of a BRCA2 mutation in a patient with recurrent thymoma: a case report
Introduction
Thymic epithelial tumors (TETs) refer collectively to a histological spectrum of rare cancers that include thymomas, thymic carcinomas, and thymic neuroendocrine neoplasms. Thymomas have a low tumor mutation burden and actionable genomic alterations are largely absent (1,2). Defects in immune tolerance increase the risk of paraneoplastic autoimmune disease, especially in patients with thymoma (1). Although thymomas can exhibit indolent clinical behavior, patients with advanced disease frequently experience disease relapse with a 10-year cumulative incidence of recurrence of 57% to 71% (1). A limited number of treatment options are available for patients with recurrent thymoma (1). Due to a low frequency of somatic mutations in recurrent thymoma (3), treatment directed toward specific genomic alterations is largely ineffective (1). Somatic mutations in the breast cancer gene 2 (BRCA2), a tumor suppressor gene that plays a key role in DNA damage repair, are present in 3% of advanced-stage TETs (3,4). Germline mutations in BRCA2 increase the risk for multiple types of cancer. Cancers harboring BRCA mutations have increased sensitivity to platinum-based chemotherapy agents (5). Poly (ADP-ribose) polymerases (PARPs) are a family of proteins involved in a number of cellular processes, including DNA repair (6). PARP inhibitors are clinically active in cancers harboring BRCA mutations, such as breast, ovarian, pancreatic and castrate-resistant prostate carcinomas (6). However, the role of PARP inhibitors in the treatment of BRCA2-mutated TETs is unclear. We present the following case of a recurrent thymoma with a BRCA2 mutation in accordance with the CARE reporting checklist (available at https://med.amegroups.com/article/view/10.21037/med-22-9/rc), which was presented at a virtual tumor board meeting of the International Thymic Malignancy Interest Group (ITMIG), an academic society consisting of an international panel of experts representing various medical specialties, and we discuss the potential role of PARP inhibitors versus cytotoxic chemotherapy for treatment.
Case presentation
A 63-year-old woman with an initial diagnosis of Tumor Node Metastasis (TNM) stage I, World Health Organization (WHO) subtype B1 thymoma experienced disease recurrence in the ipsilateral (left) costophrenic recess 30 months after initial thymectomy. She was treated with preoperative external beam radiotherapy (EBRT) to 45 Gy in 15 fractions, followed by pleurectomy, diaphragmatic resection and pericardiectomy for treatment of multiple parietal pleural metastases that were detected at the time of surgery. Histology of the resected tumors was consistent with WHO subtype B2 thymoma. However, the resection sample was not available for histology review at the ITMIG tumor board. Somatostatin receptor scintigraphy was negative. Foundation One next generation sequencing of DNA extracted from the surgical specimen demonstrated the presence of a BRCA2 mutation. Details about the BRCA2 mutation, including the variant allele frequency, and information about germline testing for BRCA2 was not available for review by the ITMIG tumor board. Family history included a sister with breast cancer and a daughter with a similar BRCA2 mutation, which was suggestive of the patient harboring a germline mutation of BRCA2.
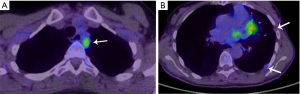
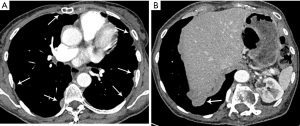
Due to microscopically positive surgical margins the patient received four cycles of post-operative chemotherapy (cisplatin, doxorubicin and cyclophosphamide). Eighteen months after surgery, a whole body 18-fluorodeoxyglucose positron emission tomography (FDG-PET)-computed tomography (CT) scan demonstrated FDG-avid recurrent disease (Figure 1A), and two small foci of disease in the pleura with low FDG uptake (Figure 1B). The patient received EBRT (36 Gy in 12 daily fractions) for treatment of disease abutting the mediastinum and the pleural deposits were monitored. Four months after the second course of EBRT, a chest CT scan demonstrated multifocal disease progression involving the left and right pleurae, with the appearance of multiple new pleural metastases (Figure 2A,2B). There was no evidence of recurrence within the previously irradiated pleura of the left costophrenic recess or development of extra-thoracic metastases.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and the accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Treatment options for recurrent thymoma include cytotoxic chemotherapy with drugs such as pemetrexed, gemcitabine, capecitabine and taxanes, targeted therapy with the mammalian target of rapamycin inhibitor, everolimus, somatostatin analogs with or without prednisone for tumors showing uptake on somatostatin receptor scintigraphy, and participation in clinical trials, when available (1). Pemetrexed is frequently used for second-line treatment of thymoma due to its ability to induce durable responses and its excellent safety profile (7). Treatment of unresectable or recurrent disease is palliative in nature.
Somatic mutations in BRCA2 have been observed in 3% of advanced-stage TETs (3). However, the functional and clinical significance of these mutations is unclear and the role of PARP inhibitors for the treatment of recurrent TETs is not clearly established. To our knowledge there is only one case reported in the literature of a patient with recurrent thymoma harboring a germline frameshift mutation in BRCA2 who experienced durable disease stabilization with the PARP inhibitor, olaparib (8). Of note, the patient had received multiple systemic therapies previously over a 10-year period with intervals of disease stability, including a two-year period while receiving an insulin-like growth factor-1 receptor inhibitor, which raises the question of the role of intrinsic tumor biology in disease stabilization rather than the effect of PARP inhibition. Recent research has also uncovered the effect of PARPs on innate and adaptive immunity as well as the tumor microenvironment (9,10). It is unclear if the immunomodulatory effects of PARP inhibition rather than synthetic lethality in the presence of an underlying BRCA2 mutation contributed to tumor control in the previously published case report.
Treatment with PARP inhibitors can cause clinically significant adverse events, including severe hematological toxicity (11). High-grade anemia was associated with treatment duration longer than 6 months, treatment with rucaparib or niraparib, and underlying ovarian cancer (11). More concerningly, an increase in the risk of myelodysplastic syndrome and acute myeloid leukemia has been described in patients treated with PARP inhibitors (12). A recent meta-analysis found a small, but increased risk of developing myelodysplastic syndrome and acute myeloid leukemia in association with PARP inhibitor therapy compared with placebo with an odds ratio of 2.63 (absolute difference in incidence: 0.73% versus 0.47%) (12). Hence, a careful assessment of potential risks and benefits is essential while considering the use of PARP inhibitors for treatment of BRCA2-mutated recurrent thymoma.
The ITMIG panel was asked for input on the optimal choice for second-line systemic therapy in a patient with a BRCA2-mutated recurrent thymoma who has previously received platinum-based combination chemotherapy, and if a PARP inhibitor is indicated due to the presence of the BRCA2 mutation. Volume reduction surgery was not considered for treatment of second recurrence due to presence multifocal disease involving bilateral pleurae. In the absence of data to support the functional and therapeutic significance of BRCA2 mutations in patients with thymoma, the potential for severe toxicity associated with PARP inhibitors, and the availability of other safe and effective treatment options, the ITMIG panel recommended the use of pemetrexed in this case for second-line treatment of recurrent thymoma and suggested that PARP inhibitors should be considered for treatment of BRCA2-mutated thymomas only as part of a clinical trial or if other treatment options have been exhausted. Further research is needed to define the role of PARP inhibitors in TETs with mutations involving DNA damage and repair genes, including BRCA2 mutations.
Acknowledgments
The patient involved in this case report provided her informed consent authorizing use and disclosure of her health information.
Funding: This work was supported in part by
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://med.amegroups.com/article/view/10.21037/med-22-9/rc
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-22-9/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-22-9/coif). EMM received an honorarium from Boehringer Ingelheim. A Rimner received grants to his institution from Boehringer Ingelheim, Pfizer, AstraZeneca, Varian Medical Systems, and Merck; and personal grants from Boehringer Ingelheim, AstraZeneca, Merck, Cybrexa, MoreHealth, ResearchToPractice, and Philips/Elekta. ACR received yearly royalties from Up-to-Date. DB received an honorarium from AstraZeneca. MS serves as an unpaid editorial board member of Mediastinum from June 2021 to May 2023. ACR serves as an unpaid Associate Editor of Mediastinum from July 2021 to June 2023. MM serves as an unpaid Associate Editor-in-Chief of Mediastinum. NT serves as an unpaid editorial board member of Mediastinum from June 2021 to May 2023. CF serves as an unpaid editorial board member of Mediastinum from July 2021 to June 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and the accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Girard N, Ruffini E, Marx A, et al. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v40-55. [Crossref] [PubMed]
- Radovich M, Pickering CR, Felau I, et al. The Integrated Genomic Landscape of Thymic Epithelial Tumors. Cancer Cell 2018;33:244-258.e10. [Crossref] [PubMed]
- Wang Y, Thomas A, Lau C, et al. Mutations of epigenetic regulatory genes are common in thymic carcinomas. Sci Rep 2014;4:7336. [Crossref] [PubMed]
- Foulkes WD, Shuen AY. In brief: BRCA1 and BRCA2. J Pathol 2013;230:347-9. [Crossref] [PubMed]
- Mylavarapu S, Das A, Roy M. Role of BRCA Mutations in the Modulation of Response to Platinum Therapy. Front Oncol 2018;8:16. [Crossref] [PubMed]
- Curtin NJ, Szabo C. Poly(ADP-ribose) polymerase inhibition: past, present and future. Nat Rev Drug Discov 2020;19:711-36. [Crossref] [PubMed]
- Gbolahan OB, Porter RF, Salter JT, et al. A Phase II Study of Pemetrexed in Patients with Recurrent Thymoma and Thymic Carcinoma. J Thorac Oncol 2018;13:1940-8. [Crossref] [PubMed]
- Principe DR, Kamath SD, Munshi HG, et al. Metastatic Thymoma Harboring a Deleterious BRCA2 Mutation Derives Durable Clinical Benefit from Olaparib. Oncologist 2020;25:301-5. [Crossref] [PubMed]
- Yélamos J, Moreno-Lama L, Jimeno J, et al. Immunomodulatory Roles of PARP-1 and PARP-2: Impact on PARP-Centered Cancer Therapies. Cancers (Basel) 2020;12:392. [Crossref] [PubMed]
- Martí JM, Fernández-Cortés M, Serrano-Sáenz S, et al. The Multifactorial Role of PARP-1 in Tumor Microenvironment. Cancers (Basel) 2020;12:739. [Crossref] [PubMed]
- Wang C, Li J. Haematologic toxicities with PARP inhibitors in cancer patients: an up-to-date meta-analysis of 29 randomized controlled trials. J Clin Pharm Ther 2021;46:571-84. [Crossref] [PubMed]
- Morice PM, Leary A, Dolladille C, et al. Myelodysplastic syndrome and acute myeloid leukaemia in patients treated with PARP inhibitors: a safety meta-analysis of randomised controlled trials and a retrospective study of the WHO pharmacovigilance database. Lancet Haematol 2021;8:e122-34. [Crossref] [PubMed]
Cite this article as: Sigurdson S, Marom EM, Rimner A, Shepherd A, Szolkowska M, Roden AC, Marino M, Tomiyama N, Ball D, Falkson C, Rajan A. The therapeutic relevance of a BRCA2 mutation in a patient with recurrent thymoma: a case report. Mediastinum 2022;6:40.


