
Principles of medical and oncological management of giant masses of the mediastinum: a narrative review
Introduction
Giant mediastinal masses include benign or malignant tumors that can develop from structures located in the mediastinum or that pass through the mediastinum during development, as well as from metastases or lymphadenopathy of malignancies that arise elsewhere in the body (1).
The mediastinum is divided into compartments, each of which is characterized by the presence of specifics structures and, consequently, neoplasms.
The prevascular or anterior compartment of mediastinum is affected by thymic tumors such as thymomas, thymic carcinomas and thymic neuroendocrine tumors, germ cell tumors, lymphomas, thyroid and parathyroid tumors and metastases. The visceral or medial compartment includes lung tumors, lymphoma and metastases. In the last mediastinal compartment, the paravertebral or posterior, neurogenic tumors arising from dorsal root ganglia/neurons adjacent to intervertebral foramina and esophageal neoplasms can be observed (2).
While mediastinal tumors are represented by well-defined histological variants originating from different structures and compartments, their clinical presentation may be similar and characterized by the same set of symptoms proper of the well-known mediastinal syndrome (MS). In fact, in 80% of cases the MS is caused by malignant neoplasms, such as lung tumors [non-small cell lung cancers (NSCLCs) and small cell lung cancer (SCLC)], in 10–18% of cases by hematological neoplasms, such as lymphomas and in 2–3% by benign causes such as goiter or thyroid hyperplasia or vascular malformations such as aneurysm or cysts (3). A summary of the different cancer types causing MS is summarized in Figure 1.
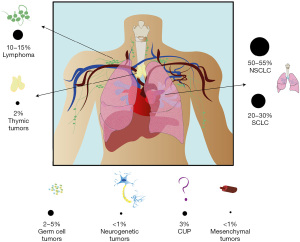
The MS is classified according to the location of the lesion and the structures involved as follows: respiratory syndrome (trachea and bronchi), vascular syndrome (arterial or venous), neurological syndrome (vagus nerve, recurrent nerves, phrenic nerve, sympathetic chain) or digestive syndrome (esophagus). Symptoms of the syndromes are associated with the anatomic structures involved and superior vena cava syndrome (VCS) and airway obstruction represent the most severe complications of MSs and are considered a medical emergency. Treatment of MS is both directed against the tumors and to the symptoms. To tackle the tumor, chemotherapy, target therapy, radiation, surgery may be used, according to the etiology of MS (4).
Symptoms are treated with supportive therapies, for instance, interventional radiology procedures such as stenting may help manage this syndrome. However, the prognosis is poor in most cases. In fact, the median survival of patients with MS ranges from 6 to 9 months and it is linked to the histology of the tumor, which therefore represents the most important prognostic factor of the MS itself (5).
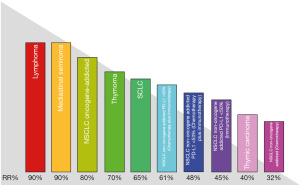
In this review we report the medical treatments of main giant mediastinal tumors, focusing our interest on the objective response rate (ORR). The ORR represents the most suitable parameter to predict the volumetric reduction of the neoplasm and, consequently, the regression of their most severe complication, the MS (Figure 2). We will also cover the supportive and symptomatic treatment of MS. We present the following article in accordance with the Narrative Review reporting checklist (available at https://med.amegroups.com/article/view/10.21037/med-21-54/rc).
Methods
We performed a literature review on December 1st, 2021. The literature search was undertaken from origin until November 30th, 2021. Only studies published in English were considered. The databases used included UpToDate, Medline and PubMed. Article types included in the search criteria were retrospective, prospective, randomized control trial, case report studies, original research, meta-analyses, abstracts, and previous related reviews. The search terms used to identify relevant articles during screening included “Mediastinal tumors, Mediastinal syndrome, Superior Vena Cava Syndrome, NSCLC (Oncogene-addicted metastatic NSCLC, Non-oncogene-addicted localized NSCLC, Non-oncogene-addicted metastatic NSCLC), Small Cell Lung Cancer (SCLC), Thymic tumors (Thymoma, Thymic Carcinoma, Thymic Neuroendocrine tumor), Lymphoma, Rare tumor (Germ Cell Tumor, Mesenchymal tumor, Neurogenic tumor), Cancer of Unknown Primary, Medical non-oncological management,” individually or in combination (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of Search (specified to date, month, and year) | December 1st, 2021 |
| Databases and other sources searched | UpToDate, Medline and PubMed |
| Search terms used (including MeSH and free text search terms and filters) | Search terms: “Mediastinal tumors, Mediastinal syndrome, Superior Vena Cava Syndrome, NSCLC (Oncogene-addicted metastatic NSCLC, Non-oncogene-addicted localized NSCLC, Non-oncogene-addicted metastatic NSCLC), Small Cell Lung Cancer (SCLC), Thymic tumors (Thymoma, Thymic Carcinoma, Thymic Neuroendocrine tumor), Lymphoma, Rare tumor (Germ Cell Tumor, Mesenchymal tumor, Neurogenic tumor), Cancer of Unknown Primary, Medical non-oncological management” |
| Timeframe | From origin until November 30th, 2021 |
| Inclusion and exclusion criteria (study type, language restrictions etc.) |
Inclusion and exclusion criteria: (I) English-language article; (II) Article types were retrospective, prospective, randomized control trial, case report studies, original research, meta-analyses, abstracts, and previous related reviews |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | The records were first screened for title or abstract by two independent reviewers (NC and FGD), and subsequently screened for full text. Debate over article selection was resolved with consensus |
Discussion
NSCLC
Lung cancer is the second most common malignancy and the first cause of death, regardless of sex, worldwide and NSCLC accounts for 80–90% of all lung cancers.
Although mediastinal lymph node involvement is common in patients with NSCLC, it often remains asymptomatic and therefore is not a frequent cause of MS.
Oncogene-addicted metastatic NSCLC
Traditionally, chemotherapy represented the cornerstone of treatment for advanced diseases, until more recent years, when the identification of the so called “oncogene-addicted” NSCLC has radically changed the therapeutic algorithm for the advanced stages of this disease. Appropriate morphological and biomolecular diagnosis are now essential to guide its therapy (6). Among these, the presence of EGFR and BRAF mutations and ALK and ROS1 rearrangements must always be assessed in patients with advanced NSCLC (stage IIIA–IV). Other agents targeting RET and NTRK rearrangements, MET exon 14, ERBB2 and KRAS G12C mutations are still available for patients carrying these targets. Oncogene-addicted diseases occur in 30% of NSCLC in western populations and are more frequent in young and non-smokers patients with adenocarcinoma histotype (6).
In the last fifteen years, several trials showed that first generation [Gefitinib (7) and Erlotinib (8)] and second generation [Afatinib (9)] tyrosine kinase inhibitors (TKIs) lead to an ORRs ranging 56–84.6% while Osimertinib, in the FLAURA trial, resulted in an ORR of 80%, matching the comparator arm represented by gefitinib or erlotinib (76%) (10).
ALK rearrangement affects 3–7% of NSCLC and occurs in young, non-smoking, male patients with adenocarcinoma histology (11). As for EGFR, ALK inhibitors are currently the main stay of systemic treatment, whit an ORR of 74–85% in patients treated with second generation TKIs and 62–74% in patients treated with first generation TKIs (12-14).
ROS1 rearrangements account for 1–2% of NSCLC cases and are specific for adenocarcinoma. Given the considerable clinicopathological overlap and homology between ROS1 and ALK, Crizotinib was also tested in ROS1-rearranged NSCLC where showed considerable clinical efficacy with an (ORR ~70%) (15,16). More recently, Entrectinib, a ROS1, NTRK and ALK inhibitor, has demonstrated clinical activity in crizotinib-naive ROS1-rearranged NSCLC, showing an even higher ORR of 77%.
Other targets with high sensitivity to the corresponding agent are BRAF, for whom the combination of dabrafenib and trametinib showed an ORR of 64% (17), MET, where capmatinib reached an ORR of 68% (in treatment-naïve patients) and 41% (in pre-treated patients) (18) and RET, with Selpercatinib and Pralsetinib achieving an ORR of 68–85% (19).
Larotrectinib, approved by FDA in 2018, based on data across a wide number of cancer types, shows an ORR of 81% among those patients with NTRK gene fusions, in a tumor-agnostic manner (20). Furthermore, Entrectinib was also approved by FDA regardless of tumor type, in patients with NTRK fusion. In STARTRK-1, 2, NG, this TRK inhibitor showed a consistent efficacy with ORR of 57% (21).
Less impressive were the results of KRAS G12C inhibitor Sotorasib, the first anti-KRAS G12C mutation, with an ORR of 54% in pre-treated patients (22).
Currently, however, not all molecularly targeted therapies can be prescribed except in the context of experimental protocols.
Non-oncogene-addicted localized NSCLC
In locally advanced non-oncogene-addicted NSCLC the options for surgery depend on the extent of the primary tumor and lymph node involvement. In fact, stages IIIA–IIIB represent a very heterogeneous group whose possible treatments are surgery followed by adjuvant chemotherapy, neoadjuvant chemotherapy followed by surgery or chemotherapy and radiotherapy, followed by durvalumab. The different therapeutic options must be evaluated within a multidisciplinary team. In patients with unresectable N2 disease, a combined treatment of chemo-radiotherapy at radical doses is indicated as the first option.
The neoadjuvant concurrent chemo-radiotherapy approach with combinations based on cisplatin plus third generation chemotherapy and RT 54–66 Gy aims to achieve presurgical downstaging, in particular at the lymph node level (23). Pathological complete response is 15–33% while lymph node clearance (N0) is 25–46% (24). Alternatively, patients who are unable to tolerate concomitant treatment or with high pathological volumes, can be treated with chemotherapy and sequential radiotherapy.
Non-oncogene-addicted metastatic NSCLC
The encouraging ORRs, seen in patients with oncogene-addicted disease, are not replicated in the non-oncogene-addicted group. Although the introduction of immunotherapy alone or in combination with standard histology-based chemotherapy, has increased the survival of these patients compared to standard chemotherapy, ORR are significantly lower than in patients with oncogene-addicted disease.
KEYNOTE-024 enrolled 305 patients with metastatic PD-L1 (TPS ≥50%) NSCLC who were assigned to either pembrolizumab as monotherapy or standard-of-care platinum-based chemotherapy. ORR was 45.5% in the pembrolizumab group compared to 29.8% in the chemotherapy group (25). Similarly, in Impower-110, atezolizumab was evaluated as first-line treatment in PD-L1 selected patients with advanced NSCLC, independent of tumor histology. This trial enrolled 572 patients with chemotherapy-naïve stage IV non-squamous or squamous NSCLC with PD-L1 expression ≥1% on tumor cells (TCs) or immune cells (ICs). ORR was 38.3% for patients treated with immunotherapy vs. 28.6% for patients treated with chemotherapy (26).
Subsequently, immune checkpoint inhibitors (ICIs) were evaluated as first-line treatment in combination with platinum-based chemotherapy. In Keynote-407 the addition of pembrolizumab improved the ORR, 57.9% vs. 38.4%. Similarly, the ORR was greater across all PD-L1 subgroups with the addition of pembrolizumab: 63.2% vs. 40.4% for PD-L1 negative, 49.5% vs. 41.3% for patients with PD-L1 of 1–49% and 60.3% vs. 32.9% for patient with PD-L1 ≥50% on TCs (27). Likewise, KEYNOTE-189 study showed that pembrolizumab plus pemetrexed-platinum significantly improved overall survival, progression-free survival, and ORR (47.6% vs. 18.9%) compared with placebo plus pemetrexed-platinum in patients with metastatic non-squamous NSCLC without sensitizing EGFR/ALK alterations, regardless of PD-L1 TPS (28).
It should be noted that an increasing amount of evidence is now questioning the efficacy of ICIs alone in patients with high tumor burden, regardless of cancer histology (29). A large number of these evidences come from lung cancer (30,31), with one study showing correlation between tumor burden and increased risk of hyperprogressive disease (meaning a sudden acceleration of tumor growth after being exposed to ICIs) in patients with large tumors (32). Considering the need of rapid shrinkage in the patients with giant mediastinal mass and that these cancers are, by definition, characterized by large volume, the use of ICIs monotherapy in such patients should be considered with caution and the combination with chemotherapy should be privileged, even in the presence of high PD-L1 expression.
In conclusion, the treatment of MS caused by NSCLC is based on molecular characterization. In fact, in oncogene-addicted disease, treatment with TKIs can determine a rapid and impressive response such as the so called “Lazarus effect” (33). Target therapy can lead to a reduction in tumor volume with consequent resolution of symptoms. In non-oncogene-addicted NSCLC, a combination treatment with ICIs and chemotherapy is recommended for metastatic disease, while concurrent or sequential chemo-radiotherapy is advised in locally advanced disease. Conversely, ICIs monotherapy should be handled with care.
SCLC
SCLC accounts for 10–15% of lung cancers. Despite the high chemosensitivity, the 10-year survival is 3.5%. Most SCLC arises from lobar or main bronchi and the most common manifestations is a large mass centrally located within the lung parenchyma or a mediastinal mass involving the hilus. Treatment of the limited-stage disease involves chemotherapy and radiotherapy with carboplatin/cisplatin + etoposide for 4 cycles and hyper-fractionated radiotherapy 45 Gy in 25–30 fractions.
Since small-cell tumors are centrally located, with mediastinal adenopathy, they account for the majority of cases of malignant SVC followed by squamous cell carcinoma, large-cell carcinoma, and non-Hodgkin’s lymphoma. Treatment options include percutaneous stent placement, corticosteroids, radiotherapy, and chemotherapy as well as thrombolytics and anticoagulation; however, the rapid start of chemotherapy, in consideration of the excellent ORR, represents the best therapeutic approach. In their work, Chan et al. evaluated the improvement of VCS’ symptoms after chemotherapy. They showed that 93% of patients enrolled in the study had significant improvement in symptoms of VCS after chemotherapy (34).
In extended disease (ED-SCLC) patients, the combination of immunotherapy plus chemotherapy showed to be superior in PFS and OS, either with durvalumab (35) and with atezolizumab (36), while chemo-ICIs combination failed to improve objective response both in terms of ORR and depth of response.
Thymic tumors
Thymoma
Thymomas are the most common tumors of anterior mediastinum and account for about 20% of mediastinal tumors. In most cases, thymomas are circumscribed masses, in other cases can be encapsulated, invade the mediastinum, or extend beyond the mediastinal pleura into lungs, pericardium, heart, large vessels, or involve the phrenic nerves. They may also be found as implants along the pleura, pericardium, and diaphragm (37).
Surgery represents the first treatment step, possibly followed by further post-operative treatments included radiotherapy and chemotherapy accordingly to the stage.
If, the lesion is judged unresectable or resectable with extended resection to other adjacent mediastinal structures, preoperative induction chemotherapy, discussed in a multidisciplinary setting, may be indicated. The suggested induction chemotherapy regimen is the combination of doxorubicin, cisplatin and cyclophosphamide (CAP regimen) for up to 4–6 cycles and the percentages of overall response rate reported in phase II studies with induction chemotherapy range from 69.6% to 77% (38). Alternatively, in patients not eligible for an anthracycline-containing chemotherapy regimen, the suggested scheme is the combination of cisplatin and etoposide.
In patients with metastatic and resectable disease, confined to the pleura and pericardium (stage IVA Masaoka or TNM IVA/M1a), induction chemotherapy is part of a multimodal approach, which can subsequently involve surgical treatment and radiotherapy. In patients with unresectable metastatic disease (Masaoka IVB and TNM IVB/M1b) or in any case not suitable for local treatment, systemic chemotherapy with palliative intent is indicated with regimens containing anthracyclines and platinum salts. They are associated with significantly higher ORRs (ORR 69.4%), when compared with regimens not containing anthracyclines (ORR 37.8%) (39,40). The suggested combination chemotherapy is CAP regimen for up to 6 cycles. Alternative regimens, represented by the combination of cisplatin and etoposide or carboplatin and taxol are considered in patients who are considered not fit for treatment with anthracyclines.
In patients progressing to first-line treatment and with good performance status, second-line chemotherapy with monochemotherapy or polychemotherapy has demonstrated an ORR ranging from 15% to 40%. Recommended treatments are the combination of gemcitabine and capecitabine, or single chemotherapy with paclitaxel, pemetrexed, ifosfamide, etoposide, gemcitabine or 5-fluoruracil (41-43). Regarding target therapies in thymoma, recent studies have evaluated anti-EGFR, anti-VEGF and Imatinib, demonstrating low ORRs (10–15%). Treatment with everolimus proved to be more promising albeit burdened with high toxicity (44). Immunotherapy with anti-PD1 and anti-PD-L1 showed ORRs of 28.6% and 29% respectively, again burdened by high severe toxicity (45), probably due to the predisposition of these patients to autoimmune disease (46).
Thymic carcinoma
Thymic carcinomas are more aggressive than thymomas; evidence of invasion of mediastinal structures is present in most of patients at diagnosis (47). As for thymomas, also in thymic carcinoma the standard treatment for resectable localized or locally advanced forms is represented by surgical treatment, followed by adjuvant chemo- and radiotherapy treatments. In patients with locally advanced unresectable disease (Masaoka III and TNM IIIA/T3 and IIIB/T4) induction chemotherapy treatment is indicated as part of a multimodal approach with curative intent, which includes a subsequent surgical and radiotherapy treatment in case of obtaining resectable disease, or definitive radiotherapy or concomitant chemo-radiotherapy. Phase II studies that evaluated the activity of induction chemotherapy treatments reported ORR rates of 69.6% to 77%. The recommended treatment is the combination of carboplatin plus paclitaxel continued for 4–6 cycles (48).
In patients with metastatic disease (Masaoka IVA–IVB, or TNM IV/M1a-b), a systemic treatment with palliative chemotherapy is recommended. The meta-analysis of Okuma et al. analyzed pooled data from 4 prospective and 6 retrospective studies, they suggest that ORR rates are not significantly different between platinum and anthracycline salt regimens (41.8%) and schemes containing platinum salts but not anthracyclines (40.9%) (39). The suggested regimen is a multi-chemotherapy with carboplatin and paclitaxel for up to 6 cycles.
In patients progressing to first-line chemotherapy and with good performance status, second-line chemotherapy is indicated. Various studies report ORR ranging from 5% to 26% with sunitinib (49), combination of gemcitabine and capecitabine, anthracycline with or without cyclophosphamide, or monochemotherapy with pemetrexed, ifosfamide, etoposide, paclitaxel or 5-fluoruracil or a rechallenge with chemotherapy regimens containing platinum salts with or without anthracyclines. Low ORR rates (10–15%) were obtained in studies evaluating anti-EGFR and anti-IGFR treatments. More promising results were obtained with imatinib, lenvatinib (ORR 39%) and everolimus. Immunotherapy with pembrolizumab also demonstrated good ORRs with ORRs of 20–22% correlated with an acceptable toxicity profile (50).
Thymic neuroendocrine tumor
Neuroendocrine carcinomas of the thymus account for 2–4% of tumors of the anterior mediastinum. Three histological subtypes have been described: well-differentiated neuroendocrine carcinoma (formerly known as “typical carcinoid”), moderately differentiated neuroendocrine carcinoma (formerly known as “atypical carcinoid”) and poorly differentiated neuroendocrine carcinoma (also known as thymic small cell carcinoma) (51). Furthermore, thymic neuroendocrine tumor may also be present in multiple endocrine neoplasia type 1 (MEN-1) syndrome. The treatment consists of total thymectomy and complete excision of the tumor, usually associated with radiotherapy and postoperative chemotherapy.
In typical advanced carcinoids, the standard treatment is somatostatin analogues. In patients with slowly progressive tumors, multiple locoregional management may represent the only anti-tumor strategy. In atypical carcinoids, first-line therapy is always based on somatostatin analogues (52). Treatment with everolimus (53), platinum salt-based chemotherapy (54), Peptide Receptor Radionuclide Therapy (PRRT) or INF alpha may be initiated following progression.
Lymphoma
A variety of lymphomas can occupy the mediastinum, either alone or as a clinically significant component of more widespread disease. The most common hematological diseases of mediastinum are represented by Hodgkin lymphoma (HL), diffuse large B cell lymphoma (DLBCL) and primary mediastinal large B cell lymphoma (PMBCL). Discovery a mediastinal mass is a common presentation of lymphomas, the mass may be asymptomatic or associated with chest pain or other symptoms.
Advanced HL is mainly treated with combination of chemotherapies. The evolution of modern cytotoxic combination regimens has been outlined in the introduction and establishment of ABVD (Doxorubicin, Bleomycin, Vinblastine and Dacarbazine) as the first-line treatment. This combination chemotherapy presents an ORR after accomplishment of the treatment of 92% (55). Even patients who are not cured with this therapy can often be rescued with alternate chemotherapy combinations, the novel antibody-drug conjugate brentuximab, or high-dose autologous or allogeneic hematopoietic stem cell transplantation (55). Also, the programmed death-1 inhibitors nivolumab and pembrolizumab have both demonstrated high response rates and durable remissions in patients with relapsed/refractory HL (56).
Diffuse large B-cell lymphoma is a common type of non-Hodgkin lymphoma (NHL), representing approximately 24% of new cases of NHL each year. The disease is aggressive, and patients typically present with rapidly enlarging lymphadenopathy and symptoms, necessitating immediate treatment. The most common up-front treatment is chemoimmunotherapy with R-CHOP regime (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), which leads to cure in approximately 50–60% of patients. Efforts to improve up-front therapy in DLBCL have combined biologic agents, including ibrutinib, bortezomib, or lenalidomide with R-CHOP with varying success (57). While most patients respond, 30–40% relapse or are unable to achieve remission with first-line treatment. In these cases, the prognosis is poor. Approximately 50% of patients with relapsed or refractory DLBCL have a response to second-line chemotherapy; up to 50% of these patients proceed to undergo autologous hematopoietic stem-cell transplantation in some settings, and of these, approximately 30–40% remain progression-free 3 years after transplantation. Patients who progress after receiving R-CHOP receive combination salvage chemotherapy. Commonly used regimens, including R-ICE, R-DHAP, R-GDP, R-GemOx, O-DHAP, O-ICE, and DR-ICE (58).
PMBCL represents a distinct clinicopathologic disease that should have a separate management approach, compared with the other subtypes of DLBCL. These diseases have an elevated cure rate with current standard approaches. Because these mediastinal lymphomas are rare and have only been described relatively recently, there are very few studies to update on optimal up-front therapy. R-CHOP and MACOP-B-like regimens followed by mediastinal radiotherapy were associated with a 5-year PFS of 75–85%. More intensive regimens, like DA-EPOCH-R without mediastinal RT, have shown very promising results. Treatment with R-CHOP resulted in a 92% ORR (with 60% CR). Approximately 10% of PMBCL patients have refractory disease and may benefit from integration of novel therapies in the frontline setting.
In conclusion, these pathologies are characterized by a high ORR and significant proportion of patients may be cured with different regimens of polychemotherapies.
Rare tumor
Germ cell tumor
Mediastinal germ cell tumors can occur as primary neoplasms in the mediastinum. In adults, the most common site in order of frequency is the anterior mediastinum. Extragonadal germ cell tumors are classified as seminomas (termed dysgerminomas in women), non-seminomatous germ cell tumors (termed non-dysgerminomas in women), mature teratomas, and immature teratomas based upon histology. They occur far more often in men than in women and usually are diagnosed between the ages of 20 and 40 years. Most patients with mediastinal germ cell tumors are asymptomatic at presentation. Common signs and symptoms of these diseases may include fever, chills, weight loss, chest pain, dyspnea, and/or superior VCS.
Mediastinal seminomas constitute approximately one-third of malignant mediastinal germ cell tumors and 2–4% of mediastinal masses. Seminomas are sensitive to both cisplatin-based chemotherapy and radiotherapy. The recommended chemotherapy for non-metastatic disease is three cycles of BEP or four cycles of etoposide plus cisplatin (EP) without bleomycin. Patients with metastatic mediastinal seminoma should be treated with four cycles of BEP chemotherapy, except for patients with lung metastases who cannot tolerate bleomycin and should receive four cycles of VIP. The remission rate with chemotherapy and resection of residual masses was 92%.
Non-seminomatous germ cell tumors of the mediastinum contain yolk sac tumor, choriocarcinoma, and embryonal carcinoma. Mediastinal non-seminomatous tumors are aggressive and often metastatic at diagnosis. A multimodality approach is generally preferred and includes chemotherapy, followed by surgery. First-choice chemotherapy consists in four cycle of VIP (59). In the study of Joel et al., partial response with elevated markers was observed in 20.5% of patients while partial response with no elevated markers in 35.2% (60). They concluded that survival outcomes among these patients have not changed dramatically, despite improvements in early diagnosis, availability of cisplatin-based chemotherapy, advancements in surgical expertise. In fact, the 5-year OS is 60%. They also showed that patients undergoing surgical resection have a better outcome when compared to those who did not undergo surgery (61,62).
Mature teratomas of the mediastinum are considered benign masses and usually tend to grow slowly. In other cases, the clinical manifestations include compression or obstruction of surrounding organs with symptoms like chest pain, cough, dyspnea, and bronchial obstruction. The treatment of mature mediastinal teratomas is surgical excision, and this is almost always curative. If only subtotal resection is possible, it is not clear that additional treatment with chemotherapy or radiation therapy offers any benefit, and observation is appropriate. Mature teratomas are relatively insensitive to both chemotherapy and radiotherapy.
Immature teratomas are rare and malignant diseases with a poor prognosis. Currently, there is no standard treatment for the mediastinal immature teratomas. Radical surgery is first-choice treatment, but the role of neoadjuvant and adjuvant chemotherapy remains uncertain. In fact, a combined approach of surgery and chemotherapy has often been recommended but the mediastinal malignant teratoma can be chemotherapy-resistant and cisplatin-based therapy may not be effective. However, for poor-risk tumors, first-line therapy usually involves chemotherapy followed by surgical resection of residual tissue (63). In fact, surgical resection of any residual mass is recommended to improve overall survival. The chemotherapy regimen includes a combination of bleomycin, etoposide, and cisplatin. Often, ifosfamide is substituted for bleomycin to avoid drug-induced lung injury. Overall complete remission rate and favourable response rate were not significantly different between the two treatments, respectively 37% of VIP vs. 31% of BEP and 63% of VIP vs. 60% of BEP (64). However, VIP chemotherapy is preferred over BEP because these patients generally require thoracic surgery after chemotherapy and are at high risk for bleomycin-related postoperative pulmonary complications like pneumonitis and pulmonary fibrosis.
Mesenchymal tumor
Soft tissue tumours arising in the mediastinum are rare; their incidence is 2–5% of all mediastinal neoplasms. Mediastinal sarcomas may rise de novo or rarely as somatic-type malignancy in a mediastinal germ cell tumour. Mesenchymal tumors of mediastinum are adipocytic, fibroblastic/myofibroblastic, fibrohistiocytic tumours, soft tissue tumours arising as somatic components in germ cell tumours, mediastinal smooth muscle, skeletal muscle, vascular, chondro-osseous, and miscellaneous tumours of uncertain differentiation, including undifferentiated sarcomas. The most common sarcomas, developing in mediastinum, are rhabdomyosarcoma and angiosarcoma.
Angiosarcoma represents a rare subcategory of soft tissue sarcomas characterized by an aggressive clinical behaviour. Usually, radical surgery and adjuvant radiotherapy represent the keystone of treatment for patients with localized disease. However, despite a proper treatment, up to 50% of patients will develop a metastatic relapse. In patients affected by advanced angiosarcoma, there is evidence of efficacy with taxane in monotherapy or in combination with anthracyclines, with gemcitabine alone or with pazopanib (65). In the study of Italiano et al., first-line single-agent doxorubicin and weekly paclitaxel seem to have similar efficacy in metastatic angiosarcomas. In fact, in doxorubicin group: 6% had complete response, 23.5% had partial response, 29.5% had stable disease, and 41% had progressive disease. In the weekly paclitaxel group: 13% had complete response, 40% had partial response, 29.5% had stable disease, 17.5% had progressive disease (66). Kollár et al. evaluated the efficacy of pazopanib, obtaining partial response rates of 20% and disease stability of 17.5%, representing a clinical benefit rate of 37.5% (67). D’Angelo et al. concluded that response rate was not significantly influenced by the type of first line therapy (25% for doxorubicin, 33% for liposomal doxorubicin, 31% for taxanes) and patients that receive anthracyclines and taxanes in combinations achieved ORR of 43% in comparison to 28% for the same agents in monotherapy (68). Recently, the development of tailored medicine has modified the systemic therapy of specific subgroups of soft tissue sarcoma, with remarkable efficacy. For instance, imatinib in advanced dermatofibrosarcoma protuberans, gemcitabine in leiomyosarcomas, trabectedin in myxoid/round cell liposarcomas, and mTOR inhibitors in perivascular epithelioid cell tumors (PEComas) (69-72).
Approximately 70% of the patients with rhabdomyosarcoma are diagnosed before the age of 10 years; in fact, is the most common soft tissue sarcoma in children, comprising 4.5% of all childhood cancer. Standard treatments of rhabdomyosarcoma include combination of chemotherapy (vincristine, actinomycin D, and cyclophosphamide/ifosfamide), radiation therapy, and surgical tumor excision. Although most patients with localized disease can be cured, the outcomes in those with metastatic or recurrent tumors remain poor. Currently, several clinical trials of immunotherapy and molecular target therapy showed efficacies in patients with soft tissue sarcomas.
Neurogenic tumor
Multicentric evidence shows that the incidence of mediastinal neurogenic tumors accounts on 4–15% of mediastinal lesions. These tumors can develop from mediastinal peripheral nerves, sympathetic and parasympathetic ganglia, and embryonic remnants of the neural tube. Neurogenic tumors are most frequent in the posterior compartment of the mediastinum (5% to 95% of all posterior mediastinal neoplasms), where they can cause neurologic symptoms by compression (55–75% of mediastinal masses) (73). The major categories of neurogenic tumors that may be encountered in the mediastinum, including schwannoma, neurofibroma, malignant peripheral nerve sheath tumors, ganglioneuroma and ganglioneuroblastoma (74). Nearly half of neurogenic tumors are asymptomatic, however, when they become larger in size, they can produce symptoms of compression, invasion, or spinal cord involvement. Most of intrathoracic neurogenic tumors are benign or low-grade malignant tumors.
Mediastinal schwannomas are benign neoplasms that originate from Schwann cells that usually affect patients of both sexes in the third and fourth decades of life. They are usually asymptomatic neoplasms but, in some cases, can cause compression and paralysis of peripheral nerves such as Pancoast syndrome or Bernard-Horner syndrome. Paragangliomas are rare mediastinal tumors that originate from the ganglia of the sympathetic nervous system and usually secrete catecholamines. Usually, radical surgery is the first-choice treatment of giant benign intrathoracic tumors. Instead, the treatment of malignant mediastinal tumors is a research field of intensive investigation. Although the 5-year survival rate is low and curative surgery is usually not possible, adjuvant chemotherapy and radiotherapy can be used for metastatic disease.
Surgical resection is the treatment of choice in a large percentage of cases of neurogenic tumors. Furthermore, considering that schwannomas are usually benign diseases; mini-invasive approaches should be performed even when they arise as multiple simultaneous lesions or in unusual locations.
Cancer of unknown primary (CUP)
Tumors of unknown origin (CUP syndrome) usually manifest with distant metastases, with different clinical manifestations based on the organ involvement with a poor prognosis in most of the cases, and a median survival of about 8–12 months. The most common manifestations of CUP syndromes are metastases in the mediastinal and axillary lymph nodes, that can provoke MS. Disseminated metastases are seen in most cases (75–85%) while solitary metastases or metastases limited to lymph nodes are only observed in 15–25% of cases (75). In case of only a solitary metastasis or the incidence of a single lymph node, a local radical surgical or radiotherapy can be carried out with curative intent (76,77). Systemic therapy based on the finding of extensive pathological characterization is recommended for widely disseminated CUP syndromes. Standards of treatment for adenocarcinomas with no indication of enteral origin, and for undifferentiated carcinomas, are combination chemotherapy of platinum (cisplatin, carboplatin) and taxane, gemcitabine, irinotecan, or platinum-free combination therapies or monotherapies (78). Overall, the response rate in real CUP, meaning those in which the origin cannot be found even with advanced techniques, is around 20% (75,79).
Medical non-oncological management
The purpose of malignant SVC syndrome’s management is to alleviate acute symptoms and treat the underlying disease. Treatment of the underlying cause depends on the histology of cancer while adjuvants medical therapy, including corticoids, diuretics and systemic anticoagulation will be administered regardless of cancer types.
Glucocorticoids (such as prednisone or methylprednisolone) may be helpful in two different settings of treatment of giant mediastinal tumors, causing MS. In the first setting, glucocorticoids can be used in steroid-responsive malignancies, such as lymphoma or thymoma, together with chemotherapy. In the other setting, high-dose glucocorticoids can minimize the risk of central airway obstruction secondary to edema in patients undergoing external beam radiation therapy and decrease the inflammatory response to tumor invasion by reducing edema surrounding the tumor (80).
Diuretics (such as furosemide) are recommended because they can reduce venous return to the heart which relieves the increased pressure and remove extra fluid from the body, although it is unclear whether small changes in right atrial pressure affect venous pressure distal to the obstruction. However, if diuretics do not alter symptoms, they should be stopped (81).
Anticoagulation is commonly used as primary prevention, but its benefit remains to be proven. In cases of proven thrombosis, systemic anticoagulation is generally recommended to limit thrombus extension (in the absence of contraindications) until definitive treatment can be undertaken (82).
Despite the wide variability depending on the underlying malignancy and the improvements of treatment, the median survival among patients who present malignant SVC syndrome remains approximately six months (83). In patients with advanced tumors and poor prognosis, palliative care should be initiated early (84).
Conclusions
In conclusion, considering the variety of pathologies that can occur in the mediastinum, a rapid histological characterization of the neoplasm is mandatory. In fact, the treatment of these neoplasms includes different approaches, sometimes used in combination, which include chemotherapy, radiotherapy, and surgery. Despite the great histological variability of mediastinal neoplasms, the clinical presentation and symptomatology is comparable and depends on the location of the mass and on the structures involved. Indeed, the main symptoms may be nonspecific such as chest pain or fever or may depend on the invasion or compression of vascular, nerve or respiratory structures, causing compression syndromes such as superior VCS. The VCS, due to its high mortality, is considered an oncological emergency and, therefore, requires effective treatments carried out urgently, evaluated in multidisciplinary meeting.
In advanced NSCLC, it is advisable to carry out molecular characterization by NGS (next generation sequencing) or similar panels which evaluate the presence of genetic alterations, highly responsive to target therapy (EGFR, ALK, ROS1, BRAF, MET and RET). Target therapies with TKIs have an extraordinary ORR and produce a rapid reduction of neoplastic mass.
Non-oncogene-addicted NSCLC, treatment with anti-PD-1 in monotherapy have lower response rate, and a combination of chemotherapy and immunotherapy should be preferred. In these cancers, with concomitant superior VCS, loco-regional treatments including surgery, radiotherapy, stenting, and medical treatment based on corticosteroids, antithrombotic and diuretics may be considered as they may provide a rapid resolution of acute symptoms. Mediastinal tumors with high chemosensitivity are SCLC, lymphomas, and seminomas while the tumors sensitive to radiotherapy thymomas, thymic carcinoma, lymphomas, seminomas, and non-seminomas germ cell tumors. Other neoplasms including thymic, neurogenic, and mesenchymal tumors show poor sensitivity to chemotherapy and radiotherapy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ryuichi Waseda and Pietro Bertoglio) for the series “Management of Giant Mediastinal Tumors” published in Mediasitinum. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://med.amegroups.com/article/view/10.21037/med-21-54/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-21-54/coif). The series “Management of Giant Mediastinal Tumors” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects for the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Su S, Colson YL. Overview of Benign and Malignant Mediastinal Diseases. In: Sugarbaker DJ, Bueno R, Colson YL, et al. editors. Adult Chest Surgery, 2e. New York, NY: McGraw-Hill Education, 2015.
- Trousse D, Avaro JP. Mediastinal tumors: introduction. Rev Pneumol Clin 2010;66:3-16. [Crossref] [PubMed]
- Bellefqih S, Khalil J, Mezouri I, et al. Superior vena cava syndrome with malignant causes. Rev Pneumol Clin 2014;70:343-52. [Crossref] [PubMed]
- Jain R, Bansal D, Marwaha RK, et al. Superior mediastinal syndrome: emergency management. Indian J Pediatr 2013;80:55-9. [Crossref] [PubMed]
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006;85:37-42. [Crossref] [PubMed]
- Dall'Olio FG, Conci N, Rossi G, et al. Comparison of Sequential Testing and Next Generation Sequencing in advanced Lung Adenocarcinoma patients - A single centre experience. Lung Cancer 2020;149:5-9. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Yang JC-H, Schuler MH, Yamamoto N, et al. LUX-Lung 3: A randomized, open-label, phase III study of afatinib versus pemetrexed and cisplatin as first-line treatment for patients with advanced adenocarcinoma of the lung harboring EGFR-activating mutations. J Clin Oncol 2012;30:abstr LBA7500. [Crossref]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Koh Y, Kim DW, Kim TM, et al. Clinicopathologic characteristics and outcomes of patients with anaplastic lymphoma kinase-positive advanced pulmonary adenocarcinoma: suggestion for an effective screening strategy for these tumors. J Thorac Oncol 2011;6:905-12. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2027-39. [Crossref] [PubMed]
- Zhu Q, Zhan P, Zhang X, et al. Clinicopathologic characteristics of patients with ROS1 fusion gene in non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res 2015;4:300-9. [PubMed]
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [Crossref] [PubMed]
- Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 2017;18:1307-16. [Crossref] [PubMed]
- Wolf J, Seto T, Han JY, et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:944-57. [Crossref] [PubMed]
- Drilon A, Oxnard GR, Tan DSW, et al. Efficacy of Selpercatinib in RET Fusion-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:813-24. [Crossref] [PubMed]
- Lin JJ, Kummar S, Tan DSW, et al. Long-term efficacy and safety of larotrectinib in patients with TRK fusion-positive lung cancer. J Clin Oncol 2021;39:abstr 9109.
- Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol 2020;21:271-82. [Crossref] [PubMed]
- Palma G, Khurshid F, Lu K, et al. Selective KRAS G12C inhibitors in non-small cell lung cancer: chemistry, concurrent pathway alterations, and clinical outcomes. NPJ Precis Oncol 2021;5:98. [Crossref] [PubMed]
- Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet 2015;386:1049-56. [Crossref] [PubMed]
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-iv21. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N Engl J Med 2020;383:1328-39. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Dall'Olio FG, Marabelle A, Caramella C, et al. Tumour burden and efficacy of immune-checkpoint inhibitors. Nat Rev Clin Oncol 2022;19:75-90. [Crossref] [PubMed]
- Dall'Olio FG, Calabrò D, Conci N, et al. Baseline total metabolic tumour volume on 2-deoxy-2-18Ffluoro-d-glucose positron emission tomography-computed tomography as a promising biomarker in patients with advanced non-small cell lung cancer treated with first-line pembrolizumab. Eur J Cancer 2021;150:99-107. [Crossref] [PubMed]
- Dall'Olio FG, Abbati F, Facchinetti F, et al. CEA and CYFRA 21-1 as prognostic biomarker and as a tool for treatment monitoring in advanced NSCLC treated with immune checkpoint inhibitors. Ther Adv Med Oncol 2020;12:1758835920952994. [Crossref] [PubMed]
- Castello A, Rossi S, Mazziotti E, et al. Hyperprogressive Disease in Patients with Non-Small Cell Lung Cancer Treated with Checkpoint Inhibitors: The Role of 18F-FDG PET/CT. J Nucl Med 2020;61:821-6. [Crossref] [PubMed]
- Conci N, Dall’Olio FG, Comellini V, et al. “Lazarus effect” in patient affected by lung adenocarcinoma carrying EGFR, CTNNB1, MET exon 11 and PIK3CA mutations treated with gefitinib. Precis Cancer Med 2020;3:23. [Crossref]
- Chan RH, Dar AR, Yu E, et al. Superior vena cava obstruction in small-cell lung cancer. Int J Radiat Oncol Biol Phys 1997;38:513-20. [Crossref] [PubMed]
- Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929-39. [Crossref] [PubMed]
- Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Safieddine N, Liu G, Cuningham K, et al. Prognostic factors for cure, recurrence and long-term survival after surgical resection of thymoma. J Thorac Oncol 2014;9:1018-22. [Crossref] [PubMed]
- Kim ES, Putnam JB, Komaki R, et al. Phase II study of a multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation therapy, and consolidation chemotherapy for unresectable malignant thymomas: final report. Lung Cancer 2004;44:369-79. [Crossref] [PubMed]
- Okuma Y, Saito M, Hosomi Y, et al. Key components of chemotherapy for thymic malignancies: a systematic review and pooled analysis for anthracycline-, carboplatin- or cisplatin-based chemotherapy. J Cancer Res Clin Oncol 2015;141:323-31. [Crossref] [PubMed]
- Loehrer PJ Sr, Chen M, Kim K, et al. Cisplatin, doxorubicin, and cyclophosphamide plus thoracic radiation therapy for limited-stage unresectable thymoma: an intergroup trial. J Clin Oncol 1997;15:3093-9. [Crossref] [PubMed]
- Palmieri G, Merola G, Federico P, et al. Preliminary results of phase II study of capecitabine and gemcitabine (CAP-GEM) in patients with metastatic pretreated thymic epithelial tumors (TETs). Ann Oncol 2010;21:1168-72. [Crossref] [PubMed]
- Gbolahan OB, Porter RF, Salter JT, et al. A Phase II Study of Pemetrexed in Patients with Recurrent Thymoma and Thymic Carcinoma. J Thorac Oncol 2018;13:1940-8. [Crossref] [PubMed]
- Bluthgen MV, Boutros C, Fayard F, et al. Activity and safety of oral etoposide in pretreated patients with metastatic or recurrent thymic epithelial tumors (TET): A single-institution experience. Lung Cancer 2016;99:111-6. [Crossref] [PubMed]
- Zucali PA, De Pas T, Palmieri G, et al. Phase II Study of Everolimus in Patients With Thymoma and Thymic Carcinoma Previously Treated With Cisplatin-Based Chemotherapy. J Clin Oncol 2018;36:342-9. [Crossref] [PubMed]
- Rajan A, Heery CR, Thomas A, et al. Efficacy and tolerability of anti-programmed death-ligand 1 (PD-L1) antibody (Avelumab) treatment in advanced thymoma. J Immunother Cancer 2019;7:269. [Crossref] [PubMed]
- Shelly S, Agmon-Levin N, Altman A, et al. Thymoma and autoimmunity. Cell Mol Immunol 2011;8:199-202. [Crossref] [PubMed]
- Eng TY, Fuller CD, Jagirdar J, et al. Thymic carcinoma: state of the art review. Int J Radiat Oncol Biol Phys 2004;59:654-64. [Crossref] [PubMed]
- Lemma GL, Lee JW, Aisner SC, et al. Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma. J Clin Oncol 2011;29:2060-5. [Crossref] [PubMed]
- Thomas A, Rajan A, Berman A, et al. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: an open-label phase 2 trial. Lancet Oncol 2015;16:177-86. [Crossref] [PubMed]
- Giaccone G, Kim C, Thompson J, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol 2018;19:347-55. [Crossref] [PubMed]
- Gaur P, Leary C, Yao JC. Thymic neuroendocrine tumors: a SEER database analysis of 160 patients. Ann Surg 2010;251:1117-21. [Crossref] [PubMed]
- Ferolla P, Brizzi MP, Meyer T, et al. Efficacy and safety of long-acting pasireotide or everolimus alone or in combination in patients with advanced carcinoids of the lung and thymus (LUNA): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol 2017;18:1652-64. [Crossref] [PubMed]
- Pavel ME, Singh S, Strosberg JR, et al. Health-related quality of life for everolimus versus placebo in patients with advanced, non-functional, well-differentiated gastrointestinal or lung neuroendocrine tumours (RADIANT-4): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1411-22. [Crossref] [PubMed]
- Spada F, Antonuzzo L, Marconcini R, et al. Oxaliplatin-Based Chemotherapy in Advanced Neuroendocrine Tumors: Clinical Outcomes and Preliminary Correlation with Biological Factors. Neuroendocrinology 2016;103:806-14. [Crossref] [PubMed]
- Shanbhag S, Ambinder RF. Hodgkin lymphoma: A review and update on recent progress. CA Cancer J Clin 2018;68:116-32. [Crossref] [PubMed]
- Armand P, Engert A, Younes A, et al. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J Clin Oncol 2018;36:1428-39. [Crossref] [PubMed]
- Liu Y, Barta SK. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol 2019;94:604-16. [Crossref] [PubMed]
- Harris LJ, Patel K, Martin M. Novel Therapies for Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Int J Mol Sci 2020;21:8553. [Crossref] [PubMed]
- Wang L, Zhao J, An T, et al. Clinical Characteristics and Outcomes of Patients With Primary Mediastinal Germ Cell Tumors: A Single-Center Experience. Front Oncol 2020;10:1137. [Crossref] [PubMed]
- Joel A, Mathew N, Andugala SS, et al. Primary mediastinal germ cell tumours: real world experience in the low middle income (LMIC) setting. Ecancermedicalscience 2021;15:1186. [Crossref] [PubMed]
- Kesler KA, Rieger KM, Hammoud ZT, et al. A 25-year single institution experience with surgery for primary mediastinal nonseminomatous germ cell tumors. Ann Thorac Surg 2008;85:371-8. [Crossref] [PubMed]
- Kesler KA, Stram AR, Timsina LR, et al. Outcomes following surgery for primary mediastinal nonseminomatous germ cell tumors in the cisplatin era. J Thorac Cardiovasc Surg 2021;161:1947-1959.e1. [Crossref] [PubMed]
- Sundararajan S, Carter YM. Mediastinal Nonseminoma. StatPearls. Treasure Island (FL): StatPearls Publishing. Copyright © 2022, StatPearls Publishing LLC., 2022.
- Nichols CR, Catalano PJ, Crawford ED, et al. Randomized comparison of cisplatin and etoposide and either bleomycin or ifosfamide in treatment of advanced disseminated germ cell tumors: an Eastern Cooperative Oncology Group, Southwest Oncology Group, and Cancer and Leukemia Group B Study. J Clin Oncol 1998;16:1287-93. [Crossref] [PubMed]
- Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol 2008;26:5269-74. [Crossref] [PubMed]
- Italiano A, Cioffi A, Penel N, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer 2012;118:3330-6. [Crossref] [PubMed]
- Kollár A, Jones RL, Stacchiotti S, et al. Pazopanib in advanced vascular sarcomas: an EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Acta Oncol 2017;56:88-92. [Crossref] [PubMed]
- D'Angelo SP, Munhoz RR, Kuk D, et al. Outcomes of Systemic Therapy for Patients with Metastatic Angiosarcoma. Oncology 2015;89:205-14. [Crossref] [PubMed]
- Rutkowski P, Van Glabbeke M, Rankin CJ, et al. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials. J Clin Oncol 2010;28:1772-9. [Crossref] [PubMed]
- Patel SR, Gandhi V, Jenkins J, et al. Phase II clinical investigation of gemcitabine in advanced soft tissue sarcomas and window evaluation of dose rate on gemcitabine triphosphate accumulation. J Clin Oncol 2001;19:3483-9. [Crossref] [PubMed]
- Grosso F, Jones RL, Demetri GD, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol 2007;8:595-602. [Crossref] [PubMed]
- Wagner AJ, Malinowska-Kolodziej I, Morgan JA, et al. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol 2010;28:835-40. [Crossref] [PubMed]
- Roden AC, Fang W, Shen Y, et al. Distribution of Mediastinal Lesions Across Multi-Institutional, International, Radiology Databases. J Thorac Oncol 2020;15:568-79. [Crossref] [PubMed]
- Rodriguez EF, Jones R, Miller D, et al. Neurogenic Tumors of the Mediastinum. Semin Diagn Pathol 2020;37:179-86. [Crossref] [PubMed]
- Pavlidis N, Pentheroudakis G. Cancer of unknown primary site. Lancet 2012;379:1428-35. [Crossref] [PubMed]
- Schmidt T, Ulrich A. Surgical options in cancer of unknown primary (CUP). Radiologe 2014;54:140-4. [Crossref] [PubMed]
- Krug D, Debus J, Sterzing F. Radiotherapeutic concepts in cancer of unknown primary site. Radiologe 2014;54:145-51. [Crossref] [PubMed]
- Hainsworth JD, Spigel DR, Clark BL, et al. Paclitaxel/carboplatin/etoposide versus gemcitabine/irinotecan in the first-line treatment of patients with carcinoma of unknown primary site: a randomized, phase III Sarah Cannon Oncology Research Consortium Trial. Cancer J 2010;16:70-5. [Crossref] [PubMed]
- Pavlidis N, Khaled H, Gaafar R. A mini review on cancer of unknown primary site: A clinical puzzle for the oncologists. J Adv Res 2015;6:375-82. [Crossref] [PubMed]
- Rowell NP, Gleeson FV. Steroids, radiotherapy, chemotherapy and stents for superior vena caval obstruction in carcinoma of the bronchus: a systematic review. Clin Oncol (R Coll Radiol) 2002;14:338-51. [Crossref] [PubMed]
- Wilson LD, Detterbeck FC, Yahalom J. Clinical practice. Superior vena cava syndrome with malignant causes. N Engl J Med 2007;356:1862-9. [Crossref] [PubMed]
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016;149:315-52. [Crossref] [PubMed]
- Morin S, Grateau A, Reuter D, et al. Management of superior vena cava syndrome in critically ill cancer patients. Support Care Cancer 2018;26:521-8. [Crossref] [PubMed]
- Varani S, Dall'Olio FG, Messana R, et al. Clinical and demographic factors associated to the place of death in advanced cancer patients assisted at home in Italy. Progress in Palliative Care 2015;23:61-7. [Crossref]
Cite this article as: Conci N, Grilli G, Dall’Olio FG. Principles of medical and oncological management of giant masses of the mediastinum: a narrative review. Mediastinum 2022;6:35.


