
Impact of the TNM staging system for thymoma
Introduction
Thymic epithelial malignancy is a heterogeneous group of rare tumors with a reported annual incidence ranging from 1.3 to 3.2 per million (1). It encompasses two major groups; thymoma and thymic carcinoma, which comprise further histologic subtypes according to the WHO Classification (2). The thymoma subtype B3 and thymic carcinoma are on the aggressive spectrum of thymic tumors, associated with unfavorable prognosis and advanced stage disease (3,4). A number of clinical and pathologic parameters such as age, stage and surgical resection with clear margin status, are also associated with prognosis (5).
Masaoka-Koga classification remains the most frequently applied clinical staging system for thymic tumors (6). This was originally proposed in 1981 and subsequently revised in 1994 to the Masaoka-Koga system (7). Unlike clinical staging systems for other types of cancer, the Masaoka-Koga system is dependent on the invasiveness of the tumor through the tumor capsule and into the surrounding tissues and organs.
To align thymic tumor staging with other types of cancer, the International Association for the Study of Lung Cancer (IASLC) and International Thymic Malignancy Interest Group (ITMIG) proposed a new tumor-node-metastasis (TNM) staging system in 2014 (Tables 1,2), which was incorporated in the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC) TNM 8th edition published in 2016 (8). They applied the same principles for thymic tumors as the IASLC had built on the work for improving the staging system in lung cancer (9). In this staging system, the T-component is based on ‘levels’ of tumor invasion. A tumor is classified in a given level if one or more structures are invaded by the tumor, regardless of whether other structures in a lower level are involved or not. Significantly tumor size is not taken into account, unlike for lung cancers. Furthermore, the IASLC/ITMIG staging system uses nodal compartments to stratify lymph node status to two groups according to proximity to the thymus i.e., anterior (prethymic) or deep (cervical or thoracic). Metastatic descriptor denotes separate pleura or pericardial nodules (M1a) and pulmonary intraparenchymal nodules or distant organ metastasis (M1b) comparable to the Masaoka-Koga system IVa and IVb.
Table 1
| Stage | Details |
|---|---|
| T1 | a: Encapsulated or unencapsulated, with or without extension into mediastinal fat |
| b: Extension into mediastinal pleura | |
| T2 | Pericardium |
| T3 | Lung, brachiocephalic vein, superior vena cava, chest wall, phrenic nerve, hilar (extra-pericardial) pulmonary vessels |
| T4 | Aorta, arch vessels, main pulmonary artery, myocardium, trachea, or oesophagus |
| N0 | No nodal involvement |
| N1 | Anterior (perithymic) nodes |
| N2 | Deep intrathoracic or cervical nodes |
| M0 | No metastatic pleural, pericardial, or distant sites |
| M1 | a: separate pleural or pericardial nodule (s) |
| b: pulmonary intraparenchymal nodule or distant organ metastasis |
IASLC, International Association for the Study of Lung Cancer; TNM, tumor-node-metastasis; T, tumour; N, node; M, metastases.
Table 2
| Stage | T | N | M |
|---|---|---|---|
| I | T1 | N0 | M0 |
| II | T2 | N0 | M0 |
| IIIa | T3 | N0 | M0 |
| IIIb | T4 | N0 | M0 |
| IVa | T any | N1 | M0 |
| T any | N0, 1 | M1a | |
| IVb | T any | N2 | M0, 1a |
| T any | N any | M1b |
IASLC, International Association for the Study of Lung Cancer; TNM, tumor-node-metastasis; T, tumor; N, node; M, metastases.
There are noticeable differences between the two staging systems: stage I only includes encapsulated tumors in the Masaoka-Koga system, whilst in the TNM system this is expanded to include extension into mediastinal fat and pleura, which corresponds to stage II in the Masaoka-Koga system. Pericardial involvement defines stage III disease in the Masaoka-Koga, but stage II in the TNM. Stage III disease in Masaoka-Koga is heterogeneous as it includes invasion into any neighboring organs, regardless of surgical resectability; this is improved in the TNM system as the classification is subdivided into more resectable stage IIIA, and less resectable stage IIIB tumors.
The aim of this study is to objectively evaluate and validate the clinical and prognostic impact of the new TNM system compared to the Masaoka-Koga staging system based on a high volume of cases treated at a single institution. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/med-21-24).
Methods
We performed a single institution-based retrospective analysis of thymomas treated at Guy’s Hospital, in London, United Kingdom. Between June 1997 and April 2017, 254 patients were diagnosed with thymic malignancy and 9 patients with thymic carcinoma were excluded. A total of 245 consecutive patients with thymoma underwent radical surgical resection, and were included in the study. None of the patients was lost to follow up completed in December 2019.
Patient characteristics and operative reports were obtained from the institutional database and medical records. All patients preoperatively received contrast-enhanced computed tomography (CT) scan of the chest. Based on pathologic findings on the resected tumors, each tumor was subtyped according to the WHO histological classification (2) and staged using the Masaoka-Koga staging system (6). Retrospectively the TNM stage in each tumor was determined, using the preoperative imaging in addition to histological, surgical, and medical reports. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). Local institutional review board (IRB) ethical consent was not required for this study, but local hospital audit committee consent was obtained. Individual consent for this retrospective analysis was waived.
Statistical analysis
Continuous data were reported as appropriate to the data distribution, while categorical data were reported with counts and percentages. All potential prognostic indices were measured at the time of operation and evaluated as categorical variables.
Survival curves were computed according to the method of Kaplan-Meier. Survival comparison between groups of patients (n=448) was performed by log-rank test. Factors that significantly affected survival in univariate analysis (at P<0.10) were tested for their independent role in multivariate analysis using the Cox proportional hazards model. The stepwise backward procedure based on the likelihood ratio was used to assess the significance of covariates included in the model. Hazard ratios and 95% confidence intervals were calculated. A P value <0.05 was considered statistically significant. All analyses were conducted using the SPSS Statistics for Windows, version 18.0 (SPSS Inc., Chicago, IL, USA) software package.
Results
Patient characteristics
The study included 245 patients (median age 62 years; 51% female) with thymoma treated with surgical resection. Patients with advanced stage tumor (Masaoka-Koga stages III and IV) frequently received neoadjuvant chemotherapy (n=18; 42.9%) with the aim to achieve a complete macroscopic resection upfront. Other patient characteristics are listed in Table 3.
Table 3
| Characteristics | Value |
|---|---|
| Gender, n (%) | |
| Male | 120 (49.0) |
| Female | 125 (51.0) |
| Age (mean; SD) (years) | 61.5 (±13.1) |
| Myasthenia gravis, n (%) | |
| Present | 36 (14.7) |
| Absent | 209 (85.3) |
| WHO classification, n (%) | |
| A | 42 (17.1) |
| AB | 100 (40.8) |
| B1 | 22 (9.0) |
| B2 | 44 (18.0) |
| B3 | 34 (13.9) |
| Complete necrosis | 2 (0.8) |
| Metaplastic | 1 (0.4) |
| Preoperative treatment, n (%) | |
| None | 220 (89.8) |
| Induction chemotherapy | 24 (9.8) |
| Radiotherapy | 1 (0.4) |
| Adjuvant treatment, n (%) | |
| None | 189 (77.2) |
| Chemotherapy | 16 (6.5) |
| Radiotherapy | 40 (16.3) |
WHO, world health organization; SD, standard deviation.
Operative data
The data collection started in 1997, and therefore predates the routine utilization of minimal access thoracic surgery; therefore, an open approach was performed in most patients (n=230). Details of the surgical procedures and postoperative data are listed in Table 4. Neo-adjuvant treatment was indicated in select patients with late-stage disease to increase operability and likelihood of complete tumour resection (R0). R0 was achieved in 74.7% of patients. A microscopic (R1: n=56; 22.9%) or macroscopic (R2: n=6; 2.4%) incomplete tumor resection was frequently documented in advanced Masaoka-Koga stages III (n=20) and IV (n=11) due to tumor infiltration into adjacent local structures. Among patients with stage II disease, 30 patients had an R1 resection (invasion of the pericardial fat with tumor present at the resection margin), and one patient with R2 resection (tumor abutting left phrenic nerve with pre-existing right phrenic injury). Adjuvant radiotherapy was used in all cases of incomplete (R1/R2) resection. All patients with stage 1 disease achieved an R0 resection.
Table 4
| Variable | n=245 (100%) |
|---|---|
| Surgical approach | |
| Sternotomy | 189 (77.1) |
| VATS | 10 (4.1) |
| Thoracotomy | 30 (12.2) |
| RATS | 5 (2.0) |
| Hemiclamshell | 6 (2.4) |
| Sternotomy and thoracotomy | 5 (2.0) |
| Type of resection | |
| Thymectomy | 217 (88.5) |
| Thymectomy and lung resection | 26 (10.6) |
| Thymectomy and vascular resection | 2 (0.8) |
| Completeness of resection | |
| R0 | 183 (74.7) |
| R1 | 56 (22.9) |
| R2 | 6 (2.4) |
| Postoperative complications | |
| Yes | 41 (16.7) |
| No | 204 (83.3) |
VATS, video-assisted thoracoscopic surgery; RATS, robotic-assisted thoracoscopic surgery.
Complete (R0) resection was associated with a median overall survival of 222 months (95% CI: 121–323 months) as compared 107 months (95% CI: 79–134 months) and 98 months (95% CI: 36–109 months) for R1 and R2 resection respectively (P<0.0001).
Stage distribution
Comparison of the two staging systems revealed that the number of stage I patients increased from 74 patients in Masaoka-Koga system to 203 in the TNM prognostic stage grouping, as expected given the broader definition of stage I disease in the TNM system. All Masaoka-Koga stage II patients (n=129) were down staged to TNM stage I. Eight patients were down staged from Masaoka-Koga stage III to TNM stage II because of pericardial involvement. The proportion of patients with stage III and IV disease was similar between the two classifications (Masaoka Koga n=42; TNM n=34). Stage distribution between the Masaoka-Koga system and TNM system is shown in Table 5.
Table 5
| Masaoka-Koga stage | IASLC/ITMIG TNM stage | Total | |||||
|---|---|---|---|---|---|---|---|
| I | II | IIIA | IIIb | IVa | IVb | ||
| I | 74 | 74 | |||||
| IIa | 117 | 117 | |||||
| IIb | 12 | 12 | |||||
| III | 8 | 21 | 1 | 30 | |||
| IVa | 11 | 11 | |||||
| IVb | 1 | 1 | |||||
| Total | 203 | 8 | 21 | 1 | 12 | 0 | 245 |
IASLC, International Association for the Study of Lung Cancer; IMTIG, International Thymic Malignancy Interest Group; TNM, tumor-node-metastasis.
The TNM stage distribution in relation to WHO histological classification is shown in Table 6. Advanced stage disease was associated with more aggressive histology subtype (P=0.0001). In our cohort only one patient was found to have nodal disease.
Table 6
| WHO histological subtype | IASLC TNM stage | Total | |||||
|---|---|---|---|---|---|---|---|
| I | II | IIIA | IIIb | IVa | IVb | ||
| A | 38 | 1 | 3 | 42 | |||
| AB | 99 | 1 | 1 | 101 | |||
| B1 | 15 | 2 | 4 | 1 | 22 | ||
| B2 | 29 | 4 | 6 | 4 | 43 | ||
| B3 | 19 | 1 | 7 | 1 | 6 | 34 | |
| Metaplastic | 1 | 1 | |||||
| Necrosis only | 2 | 2 | |||||
| Total | 203 | 8 | 21 | 1 | 12 | 0 | 245 |
WHO, World Health Organization; IASLC, International Association for the Study of Lung Cancer; IMTIG, International Thymic Malignancy Interest Group; TNM, tumor-node-metastasis.
Survival analysis
Disease-free survival
At the end of follow up, 21 patients had a recurrence of thymoma following complete resection, and 63 (25.7%) had died. The disease-free interval correlated with TNM staging (P<0.005), Masaoka-Koga (P=0.008), resection status (P=0.001) and histological subtype (P=0.002).
The median survival time was 158 months (range, 108–208 months). Advanced stage disease (III and IV) in both Masaoka-Koga (P=0.004) and TNM (P<0.0001) staging systems was associated with worse survival time as shown in Figure 1.
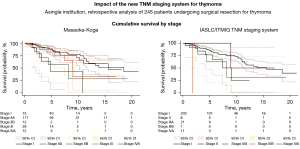
Median survival time by stage for both Masaoka and TNM staging systems was: 187 months (95% CI: 75–299 months) in Masaoka-Koga stage I; 166 months (95% CI: 87–245 months) in stage IIa, 58 months (95% CI: 17–98 months) in stage IIb; 107 months (95% CI: 87–126 months) in stage III; 53 months (95% CI: 0–111 months) in stage IVa respectively; 166 months (95% CI: 103–228 months) in TNM stage I; 107 months (95% CI: 63–258 months) in stage II; 108 months (95% CI: 69–146 months) in stage IIIA; 22 months in stage IIIB; and 98 months (95% CI: 30–65 months) in stage IVa.
Tumour size over 4 cm did not correlate with overall survival [116 (95% CI: 46–185.7) vs. 166 (95% CI: 109–222) months], but did correlate with the disease free interval [34 (95% CI: 26.7–41.2) vs. 55 (95% CI: 46–63.1) months], P=0.007.
Survival did not correlate with thymoma subtype by WHO classification (P=0.66): type A (n=42) had a median overall survival of 132 months (95% CI: 127–136 months) type AB (n=99) of 222 months (95% CI: 135–309 months), type B1 (n=22) of 107 months (95% CI: 29–184 months), type B2 (n=44) of 162 months (95% CI: 132–204 months), and type B3 (n=34) of 108 months (95% CI: 53–162 months).
At multivariate analysis including significant factors at univariate analysis only R status was an independent factor for survival, as shown in Table 7.
Table 7
| Variables | Hazard ratio | 95% confidence interval | P value |
|---|---|---|---|
| Masaoka stage | 1.025 | 0.79–1.32 | 0.851 |
| TNM stage | 1.032 | 0.78–1.36 | 0.821 |
| R status | 2.34 | 1.56–3.51 | 0.0001 |
TNM, tumor-node-metastasis.
Discussion
TNM staging system of cancers was first proposed by UICC over 50 years ago and is the most widely used staging system for most malignancies. It is a useful tool to stratify survival and to plan neo or adjuvant treatment according to the prognosis. Recently the IASLC/ITMIG proposed a new TNM staging system for thymic malignancy (8). The new staging system is based on the IASLC/ITMIG collaborative worldwide database of over 10,000 cases.
In our study we evaluate the impact on the new TNM staging compared to the Masaoka staging system in stratifying survival according to staging. They both correlate with survival but only R status was an independent prognostic factor for survival.
Early stages, i.e., stage I (n=74) and II (n=129) in Masaoka-Koga system, shifted to the stage 1 (n=203) in TNM system. Advanced stages—stage III (Masaoka-Koga: n=30; TNM: n=22) and IV (Masaoka-Koga: n=12; TNM: n=12)—remained very similar and were associated with more aggressive thymoma subtypes (B2 and B3). Despite the shifting of cases in the two systems and an increased number of stage I disease using TNM, there was no difference in the overall prognosis for surgical resection in early stage thymoma between the two systems. However, the new TNM staging system is more indicative of defining treatment modalities for advanced stage III disease as it divides tumors into generally more resectable stage IIIa and less resectable IIIb. Masaoka-Koga stage III is heterogeneous as it includes invasion of any of mediastinal pleural (T1b), pericardium (T2) and other surrounding structures (T3, T4). The new TNM system used preoperatively may therefore help to identify patients with advanced stage, but potentially resectable stage 3A disease who could benefit from induction chemotherapy.
In the TNM staging system, we found that survival for stage IV disease was superior to stage IIIB. Ried et al. (10) reported similar findings with 5-year survival of 70% for stage IV disease, and 54% for stage III. This survival benefit may be explained as mortality in thymoma is based on tumor resectability with clear margins, and it is likely that direct invasion of major mediastinal structures (TNM stage III disease) confers worse prognosis than droplet metastases (TNM stage IV disease). However, our study was limited in low numbers of patients with advanced stage III and IV disease. Further prospective studies are therefore required to assess the overall survival for TNM stage III and IV.
The T descriptor of thymic epithelial tumours in both staging classifications is based on the anatomical extent of the primary tumour, no matter the size. Fukui et al. (11) showed in a series of 154 cases that tumour size >4 cm was an independent factor for disease free survival. In our series we also found that tumour size >4 cm was significantly associated with the disease-free interval (P=0.007). This raises the possibility that size may be directly related to the risk of recurrence. If confirmed in further prospective studies then it may be that size should be taken into account in further iterations of the staging systems.
The new TNM-staging system also provides information on lymph node involvement and tumor dissemination. Whilst lymph node involvement is relatively common in thymic carcinoma, it is less frequently seen in thymoma and its impact on adjuvant therapy remains unclear. Historically lymph node sampling has not been routinely undertaken, with only suspicious nodes removed in practice. Therefore, the lymph node status in our patient cohort may not reflect actual incidence, with only one patient noted to have nodal disease. Further sub-analysis could, therefore, not be undertaken. Currently, there is little evidence of lymphatic spread and impact on survival in thymoma. A recent retrospective database analysis of a Chinese population of 1,617 patients with thymoma showed 2.2% to have nodal disease, and positive nodes were associated with decreased survival (P<0.001) (12). The IASLC/ITMIG have now published a proposed lymph node map for thymic epithelial tumors (13). Further prospective studies using routine lymph node sampling in thymoma and thymic carcinoma are required to assess any possible impact on adjuvant therapies. This lymph node data collection will allow evaluation of the thymic specific lymph node map and validate its prognostic role.
This article described single high-volume institution analysis but has obvious limitations, including a single center and retrospective nature, and exclusion of non-resected cases.
Conclusions
The new TNM staging system for thymic tumors is a clinically useful tool, which may improve selection criteria for multimodality approach and neoadjuvant treatment and offers reliable prognostic information. It can be used in the clinical practice both as a clinical and pathological staging system. In thymoma, the most important prognostic factor remains complete resection and patient’s surgery should be offered when a complete resection is achievable.
Further prospective studies are required to assess long term survival for Stage III and IV disease. It may be that stage III disease is less resectable than stage IV disease, and therefore associated with poorer survival. The role of routine lymph node dissection is currently unclear, and the impact on adjuvant therapies remains controversial. Further research is required to ascertain whether routine systematic nodal sampling should be undertaken in all cases.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/med-21-24
Data Sharing Statement: Available at https://dx.doi.org/10.21037/med-21-24
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/med-21-24). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). Local IRB ethical consent was not required for this study, but local hospital audit committee consent was obtained. Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- de Jong WK, Blaauwgeers JL, Schaapveld M, et al. Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur J Cancer 2008;44:123-30. [Crossref] [PubMed]
- Marx A, Chan JK, Coindre JM, et al. The 2015 World Health Organization Classification of Tumors of the Thymus: Continuity and Changes. J Thorac Oncol 2015;10:1383-95. [Crossref] [PubMed]
- Okumura M, Ohta M, Tateyama H, et al. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: a clinical study of 273 patients. Cancer 2002;94:624-32. [Crossref] [PubMed]
- Meurgey A, Girard N, Merveilleux du Vignaux C, et al. Assessment of the ITMIG Statement on the WHO Histological Classification and of the Eighth TNM Staging of Thymic Epithelial Tumors of a Series of 188 Thymic Epithelial Tumors. J Thorac Oncol 2017;12:1571-81. [Crossref] [PubMed]
- Weis CA, Yao X, Deng Y, et al. The impact of thymoma histotype on prognosis in a worldwide database. J Thorac Oncol 2015;10:367-72. [Crossref] [PubMed]
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [Crossref] [PubMed]
- Koga K, Matsuno Y, Noguchi M, et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int 1994;44:359-67. [Crossref] [PubMed]
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S65-S72. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Ried M, Eicher MM, Neu R, et al. Evaluation of the new TNM-staging system for thymic malignancies: impact on indication and survival. World J Surg Oncol 2017;15:214. [Crossref] [PubMed]
- Fukui T, Fukumoto K, Okasaka T, et al. Prognostic impact of tumour size in completely resected thymic epithelial tumours. Eur J Cardiothorac Surg 2016;50:1068-74. [Crossref] [PubMed]
- Gu Z, Wei Y, Fu J, et al. Lymph node metastases in thymic malignancies: a Chinese Alliance for Research in Thymomas retrospective database analysis. Interact Cardiovasc Thorac Surg 2017;25:455-61. [Crossref] [PubMed]
- Bhora FY, Chen DJ, Detterbeck FC, et al. The ITMIG/IASLC Thymic Epithelial Tumors Staging Project: A Proposed Lymph Node Map for Thymic Epithelial Tumors in the Forthcoming 8th Edition of the TNM Classification of Malignant Tumors. J Thorac Oncol 2014;9:S88-S96.
Cite this article as: Smith A, Cavalli C, Harling L, Harrison-Phipps K, Routledge T, Pilling J, King J, Bille A, Nonaka D. Impact of the TNM staging system for thymoma. Mediastinum 2021;5:32.


