Surgical management of locally advanced thymic neoplasms
Introduction
Thymic epithelial tumors (TETs) constitute a group of rare tumors. Per literature, the incidence of TETs is found to be 0.13 per 100,000 person-years (1). Paraneoplastic syndromes are common, with the most recognized being myasthenia gravis (MG). Locally advanced TETs may invade adjacent mediastinal structures, or disseminate to the pleura. There is a tendency for advanced thymomas to disseminate by shedding cells onto the pleural surface, “drop metastasis” (2). Many TETs are found incidentally on imaging. Diagnostic workup includes a combination of patient history, imaging, laboratory analysis, surgical staging and pathological examination, but diagnosis may often be challenging.
In general, MRI is preferred to CT due to its unique ability to differentiate thymic hyperplasia from neoplasm, and provides better definition of the tissue planes and invasion (3).
Surgical resection remains the standard of care, and completeness of resection is one of the most important factors for prognosis (2). WHO classification focuses on the histological staging of the thymic neoplasm and categorizes them into types A, B and C depending on the cellular structure (4,5). Anatomical (surgical) staging includes the Masaoka classification and the International Association for the Study of Lung Cancer and the International Thymic Malignancy Interest Group (IASLC/ITMIG) staging system (6). Anatomical staging incorporates the surgeon’s determination of invasion, and is considered superior to histologic staging in predicting prognosis (7). As mentioned previously, the most significant factor affecting prognosis is complete/R0 resection with en bloc resection of the TET, the thymus, surrounding mediastinal fat, and any other affected structures without violating the capsule (7).
In a retrospective study of 72 patients following resection of TETs with de novo metastasis to the pleura or pericardium, patients who had negative or microscopically positive margins, R0 or R1, were compared to those with grossly positive margins, R2 (8). Incomplete resection was a major negative predictive factor with overall survival of 5.5 vs. 11.8 years, and progression-free survival of 1.7 compared to 2.8 years of patients with R0/R1 resection. This study demonstrated that patients who underwent resection had prolonged survivals, even with disease progression. The five-year overall survival after resection was 73%, and the 10-year survival was 51%. Twenty-six patients had no evidence of disease progression since surgery, and the median survival time for this group was 7.2 years. Out of the 46 patients who had evidence of disease progression, 46% underwent at least a second resection. Additionally, a portion of the patients with disease progression were able to be observed for prolonged periods of time without intervention.
In cases of MG, whether or not a TET is present, a clinical response necessitates complete resection of all thymic tissue including all surrounding fatty tissue up to the neck and down to the diaphragm (9). For metastatic TETs with disseminated pleural disease, extended thymectomy with extrapleural pneumonectomy (ETEPP) has been described. Albeit small sample size, Ishikawa and colleagues demonstrated a 5-year survival of 75% for ETEPP compared to 16% for pleurectomy alone (10). In fact, aggressive surgical resection including ETEPP has even been advocated for patients in myasthenic crisis (11).
Based on the above discussed studies, which have all demonstrated positive benefit for resection of locally advanced TETs, we will share our technique of robotic-assisted thoracoscopic surgery (RATS) for resection of these tumors.
Surgical evaluation and treatment
We have found that after a complete diagnostic and staging workup, intraoperative thoracoscopic evaluation by the surgeon is essential prior to proceeding with resection (7). It allows visual assessment of the extent of the disease that may be otherwise undetected on imaging or via sternotomy or thoracotomy. After initial evaluation, robotic ports are placed and we complete the procedure with the robotic platform, RATS. RATS provides superior access to the mediastinum, and we consider this approach for all presentations of TET unless there is vascular invasion. If vascular invasion is present, our approach is typically median sternotomy unless the tumor is invading the aortopulmonary window, in which case a thoracotomy may provide better exposure.
We prefer single-lumen endotracheal intubation with endobronchial blocker in order to isolate the lung. Positioning of the patient depends on the type of lesion. Non-thymomatous in those with MG or tumors smaller than 4–5 cm, the patient is positioned in lateral-decubitus with 30-degree elevation of the ipsilateral chest. For larger tumors, especially with invasion into the lung, phrenic nerve, or pericardium, the individual is positioned in the lateral decubitus position with 30-degree posterior tilting. This position allows further resection of nearby structures, allows an excellent view of the aortopulmonary window and lung hilum, and gives the advantage of placing a 4th robotic arm, which would otherwise be challenging while supine.
There has been some debate on the preferred side for the procedure. However, anatomical studies have shown greater propensity for ectopic thymic tissue on the lateral side of the left phrenic nerve (12,13). For this reason, we generally prefer an approach from the left side, especially in MG patients, unless the thymoma is large enough and extends to the right side.
Steps of procedure
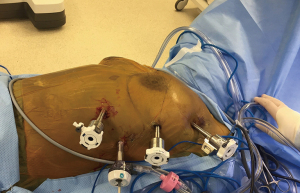
The operation begins with placement of an 8 mm port at the expected assistant port location, and assess the location and relationship of the tumor with respect to the left brachiocephalic vein, lung, phrenic nerve and sternum. Barring any evidence of unresectability, we continue with placing the remainder of the ports. With the patient in supine we use four ports: one 8-mm port at 3rd intercostal space (ICS) in the mid-axillary line, one 8-mm port at 5th ICS in the anterior axillary line for the 0-degree camera, one 8-mm port at the 7th ICS in the mid-clavicular line, and one 8-mm assistant port in the 7th ICS at the anterior axillary line (Figure 1).
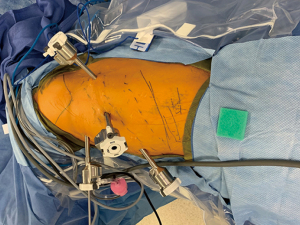
In the lateral decubitus position, we use five ports. We attempt to place all four robotic ports at 7th ICS in the anterior, middle, and posterior axillary and midclavicular lines. An 8-mm assistant port is placed at the 10th ICS anterior axillary line (Figure 2).
Robotic bipolar grasper is used in the non-dominant hand to handle tissue, with various dissecting instruments placed for the dominant hand. For TETs that are noninvasive, we use the bipolar Vessel Sealer Extend (Intuitive Surgical Inc, Sunnyvale, CA) which allows safe and hemostatic dissection. For finer dissection around the recurrent laryngeal nerve (RLN) or phrenic nerve we only use cold scissors. In the lateral position, we utilize the extra posterior port for a tip-up grasper which allows gentle retraction of the lung.
The operation begins with identification and separation of the phrenic nerve and the thymus gland. This is followed by separation of the pericardium from the thymus. The pleura of the mediastinum is then divided starting at the thoracic inlet, down to the diaphragm, which is all retrosternal. The left internal mammary vein is identified and followed to the left brachiocephalic vein. The left phrenic nerve is typically located at the junction of the left mammary vein and left brachiocephalic vein to the side of the arch of the aorta. Attention is then paid to deliver both superior thymic horns using careful sharp dissection. Controlling the thymic veins with the vessel sealer, the thymus is mobilized from the left brachiocephalic vein. Next, after opening the contralateral mediastinal pleura, the thymus gland is separated from the opposite phrenic nerve. The right phrenic nerve is usually clearly identified along the superior vena cava (SVC), running down on the pericardium. If identifying the nerves is difficult, we ask our anesthesia colleagues to inject 3 mg of intravenous indocyanine green and use infrared imaging which often facilitates identification of the adjacent phrenic nerve vessels. Once completely separated, the specimen is then placed in a tissue retrieval bag, which is sealed and left in the chest for later removal. We then complete a lymph node dissection of stations 5, 6, 3 and 10 routinely. Other stations are included selectively based on preoperative imaging and tumor invasiveness. Finally, the specimen is retrieved by extending the length of the assistant port as necessary. A flexible 24 fr blake catheter is inserted and the robot is undocked.
At the conclusion of the case, the surgeon must clearly mark the resected tumor as it is nearly impossible for pathology to otherwise understand the orientation of the specimen. The marked specimen should identify the thymic boundaries next to the SVC, pericardium, left brachiocephalic vein, and pleura bilaterally. Surgical documentation must address the extent of the resection, with any additional structures removed, lymph node stations dissected, areas of tumor violation and how the specimen was marked.
Depending on intraoperative findings, we may need to modify the above approach. For tumor invasion into the lung, a wide local excision of the lung with an endo-GIA stapler (Medtronic, Fridley, MN) with an endo-GIA stapler can be easily done when in the lateral decubitus position as detailed above.
When there is a questionable involvement of the phrenic nerve, it may be necessary to verify that the nerve is actually invaded and not displaced by the tumor. A careful, sharp dissection in close proximity to the nerve can be done. However, if the nerve is encased by the tumor, it will need to be sacrificed to ensure adequate margin. However, one must be certain that the contralateral phrenic nerve is functional and not endangered of injury with further dissection. Although unilateral phrenic nerve division can usually be well tolerated, bilateral phrenic nerve injury is a catastrophic complication.
Evaluating for pericardial invasion can also be done by creating a pericardiotomy away from the area of invasion, and then circumferentially dividing the pericardium around the tumor. When the resulting pericardial window is large, especially on the right side, we patch the defect with bovine pericardium using a running 3-0 prolene suture. The diaphragm is rarely invaded, however it is also feasible to resect any affected part of the diaphragm and repair the defect with or without mesh.
If the brachiocephalic vein is invaded, we staple it off using the endo-GIA stapler. This can also be done robotically. The only absolute indication for converting to an open procedure in our practice is for major arterial invasion, usually the innominate artery. This requires a sternotomy and vascular reconstruction.
Conclusions
TETs are a group of rare malignancies that are often slow growing and follow an indolent course. There is ample evidence that surgical resection is the mainstay of treatment for these tumors. However, the main risk of surgical resection is local recurrence. It is therefore imperative that the surgeon should achieve complete resection with negative margins. This may sometimes require extensive surgical procedures. Completeness of the tumor resection is the strongest prognostic indicator for improved outcomes. Our experience demonstrates the advantage of the robotic approach for resection of thymic tumors even when locally advanced.
Acknowledgments
Funding: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA006927.
Footnote
Provenance and Peer Review: This article was commissioned by Section Editor Zhuoqi Jia (Thoracic Department, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China). The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-21-32/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol 2010;5:S260-5. [Crossref] [PubMed]
- Abbas AE. Commentary: Extrapleural pneumonectomy during myasthenic crisis: The urge to go big or go home. JTCVS Tech 2020;2:173-4. [Crossref] [PubMed]
- Ackman JB, Wu CC. MRI of the thymus. AJR Am J Roentgenol 2011;197:W15-20. [Crossref] [PubMed]
- Okumura M, Ohta M, Tateyama H, et al. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: a clinical study of 273 patients. Cancer 2002;94:624-32. [Crossref] [PubMed]
- Rena O, Papalia E, Maggi G, et al. World Health Organization histologic classification: an independent prognostic factor in resected thymomas. Lung Cancer 2005;50:59-66. [Crossref] [PubMed]
- Kondo K, Van Schil P, Detterbeck FC, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the N and M components for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S81-7. [Crossref] [PubMed]
- Abbas AE. A New Testament for the Followers of Thymic Epithelial Tumors. Innovations (Phila) 2020;15:211-24. [Crossref] [PubMed]
- Choe G, Ghanie A, Riely G, et al. Long-term, disease-specific outcomes of thymic malignancies presenting with de novo pleural metastasis. J Thorac Cardiovasc Surg 2020;159:705-714.e1. [Crossref] [PubMed]
- Toker A, Sonett J, Zielinski M, et al. Standard terms, definitions, and policies for minimally invasive resection of thymoma. J Thorac Oncol 2011;6:S1739-42. [Crossref] [PubMed]
- Ishikawa Y, Matsuguma H, Nakahara R, et al. Multimodality therapy for patients with invasive thymoma disseminated into the pleural cavity: the potential role of extrapleural pneumonectomy. Ann Thorac Surg 2009;88:952-7. [Crossref] [PubMed]
- Iqbal F, Shokrzadeh C, Nawgiri R, et al. Extrapleural pneumonectomy with en bloc myocardial resection for advanced thymoma. JTCVS Tech 2020;2:168-70. [Crossref] [PubMed]
- Jaretzki A 3rd, Wolff M. "Maximal" thymectomy for myasthenia gravis. Surgical anatomy and operative technique. J Thorac Cardiovasc Surg 1988;96:711-6. [Crossref] [PubMed]
- Sanei B, Tabatabie SA, Bigdelian H, et al. Distribution of mediastinal ectopic thymic tissue in patients without thymic disease. Adv Biomed Res 2015;4:18. [Crossref] [PubMed]
Cite this article as: Akcelik A, Petrov R, Bakhos C, Abbas AE. Surgical management of locally advanced thymic neoplasms. Mediastinum 2022;6:10.