
Open redo thymectomy for a large recurrent thymoma in a patient with myasthenia gravis: a case report
Introduction
Although rare overall, thymomas are the most common tumors of the anterior mediastinum. Surgery is the cornerstone of therapy, while other forms of treatment play a role in patients with advanced disease (1). Over the past two decades, minimally invasive techniques for resection have been widely adopted by thymoma surgeons. At many centers, most procedures are nowadays performed as video-assisted thoracoscopic surgery (VATS) or robotic-assisted thoracic surgery (RATS). However, it is essential that surgeons retain the ability to master conventional resection via median sternotomy when an open procedure is superior to the minimally invasive approach. Here, we present the case of a myasthenia gravis patient with a large recurrent thymoma involving the pericardium, the aortic arch, and left upper lobe of the lung who underwent open redo thymectomy in stand-by of cardiopulmonary bypass. We present the following article in accordance with the CARE reporting checklist (available at https://med.amegroups.com/article/view/10.21037/med-21-25/rc) (2).
Case presentation
We report the case of a 45-year-old male patient with a history of open thymectomy for WHO type B thymoma 16 years ago, who was diagnosed with a recurrent mediastinal mass. The patient had a longstanding seropositive myasthenia gravis (MG) with leading facial and pharyngeal symptoms on first presentation, corresponding to stage IIb according to The Myasthenia Gravis Foundation of America (MGFA). Medical treatment with azathioprine had been switched to mycophenolate mofetil and prednisolone for borderline leukocytopenia and thrombocytopenia over the years, finally resulting in stable control of symptoms. For several months now, the patient had noticed increasing cough and mild thoracic tenderness on inspiration. Magnetic resonance imaging (MRI) was conducted and revealed a new-onset mediastinal mass, measuring 6.5 cm × 5.0 cm × 5.5 cm in the anteroposterior, transverse, and cranio-caudal dimensions, suspicious for local recurrence of thymoma (Figure 1). On imaging, the mass showed close contact with the large thoracic vessels, especially the aortic arch, suggesting possible infiltration of this structure. The last cross-sectional imaging had been performed 10 years after the initial surgery and had shown no evidence of recurrence.
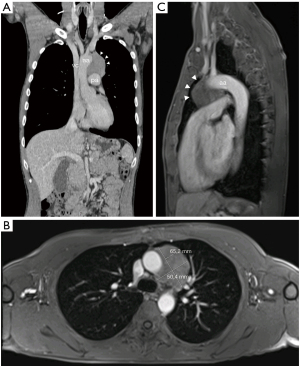
A computed tomography (CT)-guided biopsy was obtained to confirm the diagnosis. Pathology investigation of the biopsy specimen revealed a B2 thymoma with a B1 component. The case was discussed in the multidisciplinary thoracic board at our institution. Indication for 2 cycles of preoperative chemotherapy according to the PAC regimen (Cisplatin, Doxorubicin and Cyclophosphamide) followed by re-staging and subsequent resection was confirmed. Restaging after chemotherapy showed a slight decrease in tumor size but no less potential infiltration of adjacent organs. The strategy for surgery was discussed among the thoracic surgery team and the cardiac surgery team. Two main aspects had to be acknowledged. First, the patient had a history of thymectomy via median sternotomy, so that adhesions had to be expected. Second, preoperative imaging suggested potential infiltration of the aortic arch, so the need for partial aortic replacement under cardiopulmonary bypass had to be considered. Although the standard for thymectomy at our institution is RATS, we decided to perform an open thymectomy in standby for cardiopulmonary bypass.
The procedure was performed in February 2021. Intraoperatively, the tumor was safely dissected away from the aortic arch, the main pulmonary artery and the right paratracheal area; replacement of the aortic arch was not required. Due to the extent and infiltrative growth of the tumor, an additional wedge resection of the left upper lobe of the lung (segment 3), and a partial resection of the pericardium were required to ensure complete en bloc removal of the mass (Figure 2). However, during dissection, intraoperative bleeding occurred due to a short-segment tear in the aortic wall at the inner circumference.
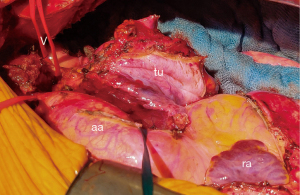
The defect was closed by suture without requiring the use of cardiopulmonary bypass. The left phrenic nerve and the left recurrent laryngeal nerve passed directly through the tumor and had to be resected. A digital chest drainage system and small size chest tubes (20 Ch) were used.
Postoperatively, the patient was monitored in the intensive care unit for 24 hours and was transferred to the normal ward on the first postoperative day. Further treatment followed ERAS (enhanced recovery after surgery) principles with an emphasis on early mobilization and adequate oral analgesia. The postoperative course was uneventful except for an episode of atrial fibrillation that was controlled with medication. The patient was discharged home in good clinical condition. No worsening of myasthenia symptoms due to surgery was observed.
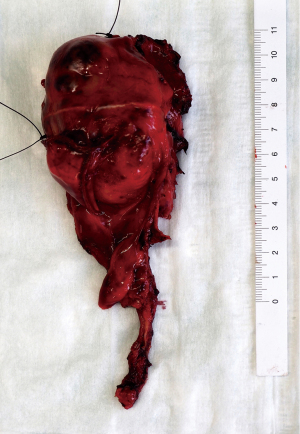
The final pathology report indicated a 6.5 cm × 5 cm × 4 cm large WHO type B2:B1 (90%, 10%) thymoma with infiltration of the lung, corresponding to the Masaoka-Koga Stage III and TNM category pT3. The resection margins were negative (Figure 3). The 4 mediastinal lymph nodes harvested were negative for malignancy. Histopathologic findings are shown in Figure 4. The case was again discussed in our multidisciplinary thoracic board and postoperative radiotherapy was recommended according to ESMO (European Society for Medical Oncology) guidelines (3). Further therapy was well tolerated by the patient. Short-term follow-up examinations showed no evidence of recurrence. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
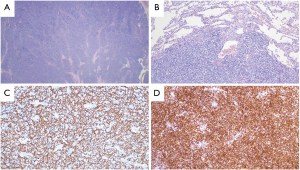
Discussion
The availability of minimally invasive techniques in thoracic surgery (VATS and RATS) has led to the increasing use of these approaches in thymoma surgery. As in other fields, this was driven by pathophysiologic considerations and pioneering spirit rather than by evidence. Today, mostly retrospective analyses indicate that minimally invasive surgery offers benefits for patients in terms of blood loss, chest tube duration, and hospital length of stay (4,5). In addition, a recent systematic review suggests that a lower rate of positive resection margins might be achieved by RATS compared with open thymectomy, however this finding must be interpreted with caution due to the nature of the underlying studies (6).
The recurrence rate after radical resection of a thymoma ranges from 10% to 30% during follow-up, with the time of recurrence being highly variable. Approximately two-thirds of recurrences are observed as pleural or pericardial lesions. Local recurrence as illustrated in the present case is far less common (7,8). One retrospective single-center analysis found recurrence in 24 of 126 patients after radical thymectomy, the most frequent type being pleural lesions (92%); local relapse was observed in only 5% of the cases (9).Like primary thymomas, recurrences are treated with radical resection when feasible; surgery is advised as the standard of care by two meta-analyses on the subject (10,11).
While minimally invasive surgery is accepted for thymoma and MG surgery in general, the optimal strategy for recurrence is controversial. Prospective data are not available, and even retrospective series or case reports on VATS or RATS resection of recurrent thymoma are rare. This is especially true for surgery after previous sternotomy where the ideal surgical access as well as the extent of resection are not defined (12). The aim of surgery in curative intent is en bloc resection with negative resection margins. After previous sternotomy, a more difficult surgical site is to be expected due to adhesions. If the resection is nevertheless performed using a VATS or RATS approach, the risk of incomplete resection must be carefully evaluated. Some authors give a tumor size threshold of 5 cm above which VATS thymectomy is not recommended (13). The present case is in accordance with this suggestion: the mass was larger than 5 cm with infiltration of the left upper lobe and the pericardium. Finally, patient safety is of utmost importance. In the case presented, iatrogenic bleeding from the aortic arch occurred during dissection. Although the goal of surgery is to prevent such an incident in the first place, it may occur, especially in complex patients with previous surgery or extensive disease. Our open surgical approach with immediate availability of cardiopulmonary bypass enabled rapid intervention and bleeding was quickly controlled. In contrast, emergency conversion of a VATS or RATS procedure to open surgery due to bleeding from the aortic arch is challenging for the surgical team and threatening to the patient. In our case, the patient recovered quickly despite the complex procedure, suggesting that in the era of fast-track surgery and ERAS, the disadvantages of open surgery carry less weight compared to minimally invasive approaches. Patients who undergo sternotomy seem to benefit from early mobilization, rapid removal of chest tubes, and adequate oral analgesia.
This case report is subject to the limitations of its genre. It represents a single and uncontrolled observation and does not allow causal conclusions or generalization. However, it may have educational value and could help improve surgical practice as it illustrates clinical reasoning in a relevant and challenging case. The complication reported represents a “no harm” event, an adverse event that occurred but did not lead to patient harm. The underreporting of adverse events in surgery, especially of no harm events, bears the risk of recurrence in different patients. Comprehensive reporting and evaluation of surgical complications, including “near misses”, remains an important issue in thymoma surgery (14).
Our patient kindly shared the following thoughts on his experience: “My recurrent thymoma was diagnosed after my myasthenia gravis symptoms worsened and my neurologist ordered an MRI. After the CT-guided biopsy, I was glad it was local recurrence and not another malignancy like lymphoma. After the diagnosis was confirmed, I was referred to a thoracic surgery center for removal. When I first met with my thoracic surgeon, he explained that many thymomas can be operated using minimally invasive techniques, but that in my case he would advise against it due to my history of prior open surgery and the possible infiltration of the aortic arch. He emphasized that open redo surgery would probably be more invasive with prolonged recovery compared to a patient who had not undergone thoracic surgery before. However, the surgeon was confident that this was the best option for me, and complete resection would be feasible. In the end, my surgery was more difficult than originally thought and I am glad that a replacement of the aorta was ultimately not necessary. However, I was surprised that, thanks to a fixed scheme of oral painkillers and physiotherapy, I had less discomfort postoperatively than I had expected. On the first post-operative day, my thoracic surgeon visited me in the normal ward and walked the corridor with me together. Based on my experience with my first sternotomy 16 years ago, I never expected this to be possible so soon and so smoothly after surgery”.
Conclusions
Minimally invasive approaches for thymectomy are rapidly gaining acceptance worldwide due to faster recovery after surgery. However, in cases with a difficult surgical site such as patients with local recurrence, a history of previous sternotomy or large tumors with infiltrative growth, open surgery may still be the better option to preserve oncologic principles and to ensure patient safety. A precise and tailored indication is crucial in these cases.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://med.amegroups.com/article/view/10.21037/med-21-25/rc
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-21-25/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-21-25/coif). EDR serves as an unpaid editorial board member of Mediastinum from November 2019 to October 2021. SP serves as an unpaid editorial board member of Mediastinum from November 2020 to October 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Khorfan R, Bharat A, Odell DD. Management and Long-Term Outcomes of Advanced Stage Thymoma in the United States. Ann Thorac Surg 2021;111:223-30. [Crossref] [PubMed]
- Gagnier JJ, Kienle G, Altman DG, et al. The CARE Guidelines: Consensus-based Clinical Case Reporting Guideline Development. Glob Adv Health Med 2013;2:38-43. [Crossref] [PubMed]
- Girard N, Ruffini E, Marx A, et al. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v40-55. [Crossref] [PubMed]
- Hess NR, Sarkaria IS, Pennathur A, et al. Minimally invasive versus open thymectomy: a systematic review of surgical techniques, patient demographics, and perioperative outcomes. Ann Cardiothorac Surg 2016;5:1-9. [PubMed]
- Agatsuma H, Yoshida K, Yoshino I, et al. Video-Assisted Thoracic Surgery Thymectomy Versus Sternotomy Thymectomy in Patients With Thymoma. Ann Thorac Surg 2017;104:1047-53. [Crossref] [PubMed]
- O'Sullivan KE, Kreaden US, Hebert AE, et al. A systematic review of robotic versus open and video assisted thoracoscopic surgery (VATS) approaches for thymectomy. Ann Cardiothorac Surg 2019;8:174-93. [Crossref] [PubMed]
- Ruffini E, Mancuso M, Oliaro A, et al. Recurrence of thymoma: analysis of clinicopathologic features, treatment, and outcome. J Thorac Cardiovasc Surg 1997;113:55-63. [Crossref] [PubMed]
- Okumura M, Shiono H, Inoue M, et al. Outcome of surgical treatment for recurrent thymic epithelial tumors with reference to world health organization histologic classification system. J Surg Oncol 2007;95:40-4. [Crossref] [PubMed]
- Haniuda M, Kondo R, Numanami H, et al. Recurrence of thymoma: clinicopathological features, re-operation, and outcome. J Surg Oncol 2001;78:183-8. [Crossref] [PubMed]
- Davenport E, Malthaner RA. The role of surgery in the management of thymoma: a systematic review. Ann Thorac Surg 2008;86:673-84. [Crossref] [PubMed]
- Hamaji M, Allen MS, Cassivi SD, et al. The role of surgical management in recurrent thymic tumors. Ann Thorac Surg 2012;94:247-54; discussion 254. [Crossref] [PubMed]
- Marulli G, Comacchio GM, Rea F. Video assisted thoracic surgery (VATS) for recurrent thymoma. Ann Cardiothorac Surg 2015;4:540-4. [PubMed]
- Fiorelli A, D'Andrilli A, Vanni C, et al. Iterative Surgical Treatment for Repeated Recurrences After Complete Resection of Thymic Tumors. Ann Thorac Surg 2017;103:422-31. [Crossref] [PubMed]
- McCafferty MH, Polk HC. Addition of “near-miss” cases enhances a quality improvement conference. Arch Surg 2004;139:216-7. [Crossref] [PubMed]
Cite this article as: Galata C, Porubsky S, Dohle DS, Karampinis I, Stamenovic D, Roessner ED. Open redo thymectomy for a large recurrent thymoma in a patient with myasthenia gravis: a case report. Mediastinum 2022;6:8.


