
Mediastinal mass in a young man with a previous history of dermatofibrosarcoma protuberans: metastatic disease or different entity—a case report
Introduction
Dermatofibrosarcoma protuberans (DFSP) is a relatively rare low-grade malignant tumor. Estimates of the overall incidence of DFSP are 0.3 to 0.5 cases per 100,000 persons per year. DFSP has been described in all age groups and usually presents in the fourth decade of life. There are similar frequencies in men and women and large series suggest a higher frequency in blacks than in whites (1-4). DFSP occurs predominantly on the trunk. It is marked by locally invasive growth and standard treatment consists of wide surgical excision (1,2).
Metastases from DFSP are extremely rare. If present, metastatic disease usually presents as pulmonary nodules (3). However, when other lesions in these patients are detected during staging, they are most likely to be related to a second primary or other non-metastatic origin.
On imaging studies, a mediastinal mass with soft tissue component, fat attenuation and calcifications is very suggestive of a teratoma. In general, benign teratomas comprise two-thirds of all mediastinal germ cell tumors. Teratomas are classified as mature and immature teratomas and teratomas with malignant transformation. The latter is currently being described by the World Health Organization as teratoma with somatic-type malignancy (5). These tumors are extremely rare in the prevascular mediastinum. Resection is the treatment of choice (6). However, if a teratoma with somatic-type malignancy is unresectable or has metastasized, they are generally incurable. We present the following case in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/med-21-18).
Case presentation
A 34-year-old man presented to the clinician with a longstanding cutaneous mass-like lesion in the lower back with maximal diameters of 3.3 cm × 2.9 cm × 4.4 cm. On MRI (not shown) a vascular, polylobular tumoral mass was seen. Presurgical biopsy was performed and histopathologic examination and molecular testing with FISH confirmed the diagnosis of DFSP. The mass was totally resected, with histopathological confirmation of the preoperative diagnosis and complete resection of the tumor (R0 resection).
Although the likelihood of metastatic disease in dermatofibrosarcoma protuberans is low, for reasons of staging a computed tomography (CT) of the chest was performed. CT showed no evidence of pulmonary metastatic disease, but showed a 4.0 cm × 4.9 cm × 4.7 cm- large mass in the prevascular mediastinum. This mass was well demarcated and heterogenous. There were no signs of local aggressive appearance, nor signs of vascular or mediastinal invasion. The mass showed areas with different densities, including a soft tissue component, areas with negative Hounsfield units (HU) consistent with fat, and some foci of calcification (Figure 1). A hilar adenopathy was noted on the right side (station 10R) (Figure 2). Tumor markers alpha-fetoprotein (AFP) and human chorionic gonadotropin (hCG) were normal (<1 µg/L).
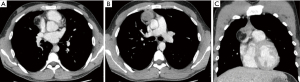
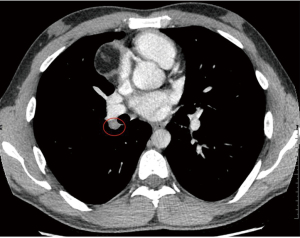
Metastases from DFSP are extremely rare. Lymph node and lung metastases are reported in less than 5% of cases. Findings in our patient were found unlikely to be related to the DFSP and to be related to a second primary. The CT-imaging features of the tumor were highly suggestive of a teratoma. Since the likelihood of metastatic disease seamed very unlikely, the mass was handled as a probable primary benign lesion and subsequently treated as such.
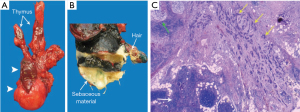
Transthoracic robotic surgery was performed with a thymectomy and tumorectomy. Systematic hilar lymph node dissection is not routinely part of surgical procedures for mediastinal masses with presumably benign nature. In this case, decision for hilar lymph node resection was related to the finding on CT of a slightly heterogeneous and enlarged right hilar lymph node. Adhesions of the tumor with the right upper lobe, made it necessary to perform a wedge-shaped resection of a portion of the upper lobe of the right lung. The histopathological examination of the excised tumor (with measurements 8.0 cm × 3.0 cm × 13.0 cm) showed residual thymus and a relatively well defined large mass with a more dense component (Figure 3A). Macroscopic examination showed sebaceous material and hair, indicating the diagnosis of a mature teratoma (Figure 3B). Photomicrograph (Hematoxylin-eosin stain, original magnification ×20) shows part of the residual thymus as well as a tumor composed of irregular sheets and glandular structures, consistent with a poorly differentiated adenocarcinoma from an intestinal type (Figure 3C). Histopathologic examination of the hilar adenopathy showed the same findings as the tumor component of the mediastinal mass. PET scan was performed for staging of the mediastinal mass, after the surgical procedure and after the unexpected diagnosis of a malignant teratoma. Brain magnetic resonance imaging (MRI) was not performed as part of the staging process. Eight months after diagnosis the patient encountered neurological symptoms in legs and feet. To rule out metastatic brain disease as cause for these neurological symptoms, brain MRI was performed. To treat any possible mediastinal micrometastases, the patient was treated with adjuvant radiotherapy of 60 Gray (Gy) in 30 fractions. Six months after the end of the adjuvant radiotherapy follow up CT-imaging showed tumor disease progression of the teratocarcinoma with pleural metastases and lung metastases. Palliative chemotherapy with folinic acid, fluorouracil and oxaliplatin (FOLFOX) was started. In course of the treatment, different treatment regiments with FOLFOX and folinic acid, fluorouracil and irinotecan (FOLFIRI) were administered. After one year the therapy was switched to FOLFIRI and afterwards because of disease progression again FOLFOX. The patient died two years and four months after presentation.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Informed consent was waived.
Discussion
Prevascular mediastinal tumors most commonly originate from the thymus or lymph nodes (7). Radiological differential diagnosis of these tumors is well known with the “4 T’s”: thymic tumors, thyroid masses, (terrible) lymphoma and teratoma. There are three large groups in the differential diagnosis: a primary neoplasm, a secondary neoplasm and a glandular enlargement. The primary neoplasms can be of thymic origin, a mediastinal lymphoma, a germ cell tumor or a neurogenic neoplasm (8). Lymphadenopathies are secondary, metastatic neoplasms of lymphoproliferative disorders or are related to thoracic or extrathoracic primary malignancies. Glandular thymic or thyroid enlargement may be responsible for a large prevascular mass. The presence of macroscopic fat, as in our case, is a determining factor for the diagnosis (9). This morphological aspect narrows down the list of differential diagnoses to germ cell tumors, thymolipoma, lipoma, liposarcoma, and Morgagni hernia (7). When there is a combination of fat, calcifications and cartilage, diagnosis of a teratoma can be suggested on imaging (9).
Mediastinal teratomas are extragonadal germ cell tumors composed of elements of all three germinal cell layers: mesoderm, endoderm and ectoderm. They are most commonly found in the prevascular mediastinum, but cases of a visceral and paravertebral compartment origin have been reported (10). They usually occur during the second to fourth decade of life (9). In the past teratomas were classified as mature, immature and teratomas with malignant transformation. The latter is currently being described by the World Health Organization as teratoma with somatic-type malignancy. These tumors are extremely rare in the mediastinum (6,11).
Patients with mature teratomas are often asymptomatic, although symptoms such as chest pain, cough, dyspnoea and bronchial obstruction can occur due to compression and obstruction of adjacent structures. In rare cases, teratomas can be complicated by rupture into the lung, pleural space or pericardium (7,8,12).
The imaging appearance of teratomas varies with the content. Both CT and MRI can clearly depict the intrinsic elements of a teratoma. The typical findings on CT are a unilateral sharply marginated lobulated heterogeneous mass in the prevascular mediastinum, with components of soft-tissue, fluid, fat and calcifications. Fat is found in 76%, calcium in 53% and fluid in 88% of cases. Combination of all three findings is found in 39% (9). A fat-fluid-level can be present. A soft-tissue component is always visualized and can have different morphological features (11,12). MRI shows a heterogeneous signal intensity as a result of the variation of internal components. The more solid components that are present in a germ cell tumor, the higher the likelihood that it is malignant. Data on imaging findings of teratomas with somatic-type malignancy are rare and mainly limited to case reports. Mustafa et al. published a case of a 21-year-old man with a malignant teratoma with a noncalcified, heterogeneous mediastinal mass with extension to the right upper lobe and pleura (6). Another case report by Habougit et al. showed a prevascular mediastinal mass with imaging features of a heterogeneous, solid and cystic lesion (13). Calcifications were present throughout the lesion and after injection of contrast there was enhancement of the solid part. These imaging features don’t really differ from those of ‘benign’ teratomas. When the typical findings of a teratoma are present, diagnosis is straightforward. In our case, the presence of an associated adenopathy could have alerted radiologists and clinicians to the possible malignant nature of the lesion. Both morphology of the mediastinal mass and the histology of the first malignancy (DFSP) made a metastatic origin of the mediastinal mass highly unlikely.
Laboratory findings have limited use in differentiating a benign from malignant etiology. An elevation of AFP and beta-hCG is not uncommon (6,8). In patients with mature teratomas tumor markers are almost always negative. In 80–90% however of malignant germ cell tumors AFP and/or beta-hCG are elevated (5).
Clinical, laboratory and radiological findings cannot reliably predict the malignant component. Diagnosis is often only made after resection. Very large and heterogeneous lesions, chest wall involvement, associated adenopathy, pleural fluid, pulmonary nodules or other signs of metastatic disease can all point to a possible malignant nature (13).
There are many histological subtypes possible in case of malignant transformation. The malignant component is often epithelial (carcinomatous) or a soft tissue malignant component. The most common soft tissue subtype is sarcoma, especially rhabdomyosarcoma, followed by adenocarcinoma and primitive neuroectodermal tumors. A final option is a nervous system tumor for example glioblastoma (6). Data on the frequency of these subtypes is lacking.
Surgical resection is the treatment of choice (7,8,11,14). Carcinomatous transformation leads to adhesions with the surrounding tissues, which makes it very difficult to perform a R0 resection. Robot assisted thoracic surgery (RATS) is the preferred surgical technique in our hospital for anterior mediastinal masses. Although large studies are lacking, RATS has been increasingly used worldwide as safe and feasible surgical technique (15-17). In our routine practice, lymph nodes that are reported on the preoperative CT as being enlarged or morphologically suspicious are resected. When surgery is performed on a prevascular mass, locoregional lymph nodes that are encountered during the procedure, are also being resected and send for histopathologic examination. The role of adjuvant chemotherapy or radiotherapy is limited. Chemotherapy based on histology of the somatic cell type has been advocated especially in patients with unresectable malignant transformation (6,14). Donadio et al. published a retrospective study of 12 cases which showed the benefit of systemic chemotherapy in a minority of patients (18). Another case report by Lin et al. showed a response from FOLFIRI chemotherapy based on the transformed histology of a colonic-type adenocarcinoma in the teratoma (19). Unfortunately, the overall prognosis is extremely poor.
Although this entity is very rare, when radiologists and clinicians encounter a mediastinal mass with typical imaging features of a benign mature teratoma, they should not forget the possibility of a malignancy. Fluorine 18 fluorodeoxyglucose (FDG) PET is not advised for mediastinal masses with a probably benign nature on imaging. However, when there is any indication of possible associated malignancy, FDG-PET-imaging may have a role to exclude or rule out distant metastatic disease and aid in preoperative planning.
Conclusions
Knowledge of classic metastatic patterns is essential in imaging the oncologic patient. In a patient with typical imaging findings of a mature teratoma in the prevascular mediastinum, associated findings such as adenopathy and/or pulmonary nodules should alert the radiologist to the possibility of a teratoma with somatic-type malignancy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/med-21-18
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/med-21-18). AD received honoraria for Presentation for Ipsen (not related to this manuscript). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). The informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rouhani P, Fletcher CD, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S. : an analysis of 12,114 cases. Cancer 2008;113:616-27. [Crossref] [PubMed]
- Kreicher KL, Kurlander DE, Gittleman HR, et al. Incidence and Survival of Primary Dermatofibrosarcoma Protuberans in the United States. Dermatol Surg 2016;42:S24-31. [Crossref] [PubMed]
- Rutkowski P, Debiec-Rychter M. Current treatment options for dermatofibrosarcoma protuberans. Expert Rev Anticancer Ther 2015;15:901-9. [Crossref] [PubMed]
- Thway K, Noujaim J, Jones RL, et al. Dermatofibrosarcoma protuberans: pathology, genetics, and potential therapeutic strategies. Ann Diagn Pathol 2016;25:64-71. [Crossref] [PubMed]
- WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. International Agency for Research on Cancer. 2015:412.
- Mustafa OM, Mohammed SF, Aljubran A, et al. Immature mediastinal teratoma with unusual histopathology: A case report of multi-lineage, somatic-type malignant transformation and a review of the literature. Medicine (Baltimore) 2016;95:e3378. [Crossref] [PubMed]
- Patel IJ, Hsiao E, Ahmad AH, et al. AIRP best cases in radiologic-pathologic correlation: mediastinal mature cystic teratoma. Radiographics 2013;33:797-801. [Crossref] [PubMed]
- Takahashi K, Al-Janabi NJ. Computed tomography and magnetic resonance imaging of mediastinal tumors. J Magn Reson Imaging 2010;32:1325-39. [Crossref] [PubMed]
- Moeller KH, Rosado-de-Christenson ML, Templeton PA. Mediastinal mature teratoma: imaging features. AJR Am J Roentgenol 1997;169:985-90. [Crossref] [PubMed]
- Lee KS, Im JG, Han CH, et al. Malignant primary germ cell tumors of the mediastinum: CT features. AJR Am J Roentgenol 1989;153:947-51. [Crossref] [PubMed]
- El Mesbahi O, Terrier-Lacombe MJ, Rebischung C, et al. Chemotherapy in patients with teratoma with malignant transformation. Eur Urol 2007;51:1306-11; discussion 1311-2. [Crossref] [PubMed]
- Choi SJ, Lee JS, Song KS, et al. Mediastinal teratoma: CT differentiation of ruptured and unruptured tumors. AJR Am J Roentgenol 1998;171:591-4. [Crossref] [PubMed]
- Habougit C, Yvorel V, Sulaiman A, et al. Mediastinal Mature Teratoma With Malignant Carcinomatous Transformation (Somatic-Type Malignancy) With Metastatic Course. Int J Surg Pathol 2015;23:682-4. [Crossref] [PubMed]
- Schaefer IM, Zardo P, Freermann S, et al. Neuroendocrine carcinoma in a mediastinal teratoma as a rare variant of somatic-type malignancy. Virchows Arch 2013;463:731-5. [Crossref] [PubMed]
- Melfi F, Fanucchi O, Davini F, et al. Ten-year experience of mediastinal robotic surgery in a single referral centre. Eur J Cardiothorac Surg 2012;41:847-51. [Crossref] [PubMed]
- Willems E, Martens S, Beelen R. Robotically enhanced mediastinal teratoma resection: a case report and review of the literature. Acta Chir Belg 2016;116:309-12. [Crossref] [PubMed]
- Chen K, Zhang X, Jin R, et al. Robot-assisted thoracoscopic surgery for mediastinal masses: a single-institution experience. J Thorac Dis 2020;12:105-13. [Crossref] [PubMed]
- Donadio AC, Motzer RJ, Bajorin DF, et al. Chemotherapy for teratoma with malignant transformation. J Clin Oncol 2003;21:4285-91. [Crossref] [PubMed]
- Lin C, Du Y, Li Y, et al. Superior mediastinal mature cystic teratoma with gastrointestinal adenocarcinoma transformation: Report of a case. Oncotarget 2016;7:38392-7. [Crossref] [PubMed]
Cite this article as: Reyntiens P, Driessen A, Rasschaert M, Snoeckx A. Mediastinal mass in a young man with a previous history of dermatofibrosarcoma protuberans: metastatic disease or different entity—a case report. Mediastinum 2021;5:35.


