Tracheal laceration causing important post-intubation delayed subcutaneous emphysema and ventilatory deterioration in a COVID-19 patient with severe rheumatoid arthritis: a case report
Introduction
The etiology of COVID-19 infection-associated pneumomediastinum and surgical emphysema have been widely reported and discussed in the literature (1,2).
In addition to the well-known iatrogenic causes such as instrumentation and barotrauma, alternative contributing factors should be investigated in the presence of systemic inflammatory diseases, which constitute airways vulnerable to inflammatory manifestations (3-5).
We report the case of COVID-19 patient with underlying severe rheumatoid arthritis who developed delayed surgical emphysema secondary to a tracheal laceration in a context of underlying active and severe rheumatoid arthritis. This case highlights the diagnostic and therapeutic challenges of patients who develop unexplained surgical emphysema and ventilatory deterioration with complicated COVID-19 infection and additional systemic inflammatory co-morbidities. We present the following article in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/med-21-12).
Case presentation
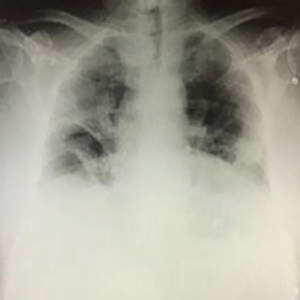
A 68-year-old male with past medical history significant of RA (on Methotrexate and Sulfasalazine), polycythaemia, high BMI with performance status of 1, presented with one week history of fever and cough, lymphopenia and raised C-reactive protein (CRP) accompanied by bilateral peripherally-dominant opacities on chest X-ray (Figure 1).
He subsequently tested positive for COVID-19 and was admitted to ICU two days later with type 1 respiratory failure. Initially he was treated with Non-invasive ventilation (NIV) but due to further deterioration, he required intubation and ventilation for adequate respiratory support and mild inotropic support. Hence, the intubation was carried out as planned by an expert physician without any immediate complications and he was ventilated on SIMV mode with PEEP of 12, FiO2of 60% on which he was able to maintain his PaO2 of at least 8. Otherwise his cardiovascular and renal function were stable requiring only minimal vasopressor support. There was also acceptable inflammatory response to pre-emptive broad-spectrum antibiotics cover.
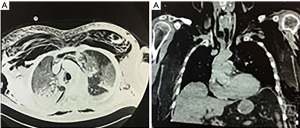
Subsequently, and nearly 2 days later it became apparent that he developed a substantial surgical emphysema over the face, front of upper chest and back, shoulder and upper arms which was progressive. Additionally, oxygen and ventilator requirements were increased needing FiO2 of 100% and PEEP 16 to maintain previously described respiratory parameters. Clinical examination and urgent chest X-ray did not support any deterioration from the COVID-19 previously noted infiltrates neither provided a plausible cause for the surgical emphysema (Figure 2A). Therefore, an urgent CT thorax was carried out which demonstrated significant pneumomediastinum and subcutaneous emphysema with air under diaphragm. There was no suspicion to suggest gastrointestinal tract perforation. Additionally, no pneumothorax was diagnosed and the previously noted COVID-19 infiltrates were stable (Figure 2B).
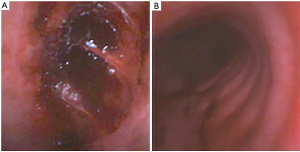
In view of increased respiratory requirements and lack of imaging evidence of any cause for the surgical emphysema and the clinical deterioration, a flexible bronchoscopy was performed, at high suspicion, which exhibited glassy like residue/laceration at 5 o’clock position approximately at 6th tracheal ring measuring 2 cm × 2 cm, possibly from previous clot or laceration. No other signs of penetration through the airways were noted (Figure 3).
The most probable cause of his deterioration initially was thought to be secondary to trauma because of his previous intubation but based on its delayed presentation, the fact that the intubation was uneventful and that it was performed by a specialist, a ulcerative lesion related to the active disease process (COVID-19 and/or RA) could not be overlooked, although biopsies were not performed because of the deteriorated condition of the patient and hence no direct evidence of this could be provided. It seems more probable however, that a trauma to the trachea was caused during intubation which was sealed by the endotracheal tube balloon which got moved later on, despite the fact that the patient was not turned to prone position as he has been unwell for this.
Next, the endotracheal tube position was adjusted (i.e., positioned more peripherally inside the trachea closer to the carina past the laceration under direct vision) and bilateral pectoral fasciotomies were performed with application of two negative suction dressings pumps (Vacuum Assisted Closure – VAC), which helped improve the extent of surgical emphysema. His O2 requirements and ventilation improved importantly and a conservative treatment for his tracheal laceration was decided based on the situation and his clinical condition.
The requisite thus far had been a single organ support. However, a few days later, patient’s clinical condition deteriorated again by developing atrial fibrillation (AF) and acute kidney and liver injury with hemodynamic compromise. He required increased vasopressor support with moderate doses of noradrenaline, pharmacological cardioversion of AF and hemofiltration was also performed. Despite multi-organ support and accepted management of COVID-19 pneumonitis at the time, his respiratory function deteriorated with increasing oxygen requirements, an increased alveolar to arterial oxygen gradient and worsening of radiological findings of acute respiratory distress syndrome (ARDS) secondary to COVID-19 pneumonitis.
Unfortunately, the patient did not recover from multi-organ failure and subsequent to multidisciplinary discussion and family involvement, the difficult decision was made to withdraw organ support on the tenth day of critical care admission in the context of worsening multi-organ failure (lung, cardiovascular and renal) caused by COVID-19 infection in a immunosuppressed state.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s next of kin.
Discussion
We herein present a case of a middle-aged man with a background of severe RA who was admitted to ICU for respiratory support due to COVID-19 infection and then developed delayed surgical emphysema and significant ventilation deterioration due to a tracheal laceration rather than lung collapse/pneumothorax. This complication should be suspected by physicians treating respiratory deterioration with surgical emphysema in patients with important underlying disease in this newly emerged viral infection.
COVID-19 infection is recognized as a causing factor of central and upper airway inflammation and oedema leaving patients potentially vulnerable to trachea-bronchial injury from intubation particularly in emergency settings (1).
A common cause of pneumomediastinum/subcutaneous emphysema in intubated patients is increased airway pressures because of mechanical ventilation although underlying diseases such as infection, sarcoidosis or rheumatoid arthritis can rarely also cause it (2). However in our case, in context of underlying rheumatoid arthritis, absence of apparent intubation difficulty and lack of pneumothoraces has led to the assumption that an overall inflammatory state of the airways along with increased pressures to achieve ventilation in this Covid-19 situation could have caused this delayed ventilatory deterioration and surgical emphysema, although a direct trauma from intubation seems could have preceded this setting.
The bronchoscopy findings of tracheal lacerations raises the question for the inflammation of upper airway secondary to underlying rheumatoid arthritis which is supported by the laryngoscopic findings (4). Up to 75% of a range of laryngeal manifestations can be found which included mucosal odema, inflammation of arytenoids, presence of rheumatoid nodules in larynx and pharynx (3). A case of rheumatoid arthritis associated with scattered lesions in the trachea with intact intraluminal membranes was also reported and the findings were thought to be consistent with early tracheal manifestation of necrotising tracheo-bronchitis (4).
In terms of the management of pneumomediastinum and subcutaneous emphysema in our case, conservative approach was utilized in context of the clinical status of the patient. A few cases of COVID -19 infection complicated with subcutaneous emphysema and managed conservatively with positive outlook have been reported (5,6). Use of negative pressure wound dressings for subcutaneous emphysema has had favorable outcomes and should be considered in similar cases (7,8).
Conclusions
This is a case of a COVID 19 patient with a background of severe active RA who developed delayed surgical emphysema and respiratory deterioration 2 days after an uneventful and elective intubation. Lack of clinical and imaging findings triggered a bronchoscopy during which a tracheal laceration was identified. Conservative management with endotracheal tube repositioning and negative pressure drainage proved adequate to treat the issue. Delayed eroding processes because of inflammation within the airways should be highly suspected when treating COVID-19 patients with underlying inflammatory co-morbidities.
Acknowlegments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/med-21-12
Peer Review File: Available at https://dx.doi.org/10.21037/med-21-12
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/med-21-12). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s next of kin. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wali A, Rizzo V, Bille A, et al. Pneumomediastinum following intubation in COVID‐19 patients: a case series. Anaesthesia 2020;75:1076-81. [Crossref] [PubMed]
- Zhou C, Gao C, Xie Y, et al. COVID-19 with spontaneous pneumomediastinum. Lancet Infect Dis 2020;20:510. [Crossref] [PubMed]
- Bejvan SM, Godwin JD. Pneumomediastinum: old signs and new signs. AJR. AJR Am J Roentgenol 1996;166:1041-8. [Crossref] [PubMed]
- Hamdan AL, Sarieddine D. Laryngeal manifestations of rheumatoid arthritis. Autoimmune Dis 2013;2013:103081 [Crossref] [PubMed]
- Kajikawa S, Noda K, Nozaki Y. Necrotizing tracheobronchitis associated with rheumatoid arthritis. Respir Med Case Rep 2016;20:31-33. [Crossref] [PubMed]
- Sun R, Liu H, Wang X. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J Radiol 2020;21:541-4. [Crossref] [PubMed]
- Towe C, Solomon B, Donington JS, et al. Treatment of recalcitrant subcutaneous emphysema using negative pressure wound therapy dressings. BMJ Case Rep 2014;2014:bcr2014205577 [Crossref] [PubMed]
- Johnson CH, Lang SA, Bilal H, et al. In patients with extensive subcutaneous emphysema, which technique achieves maximal clinical resolution: infraclavicular incisions, subcutaneous drain insertion or suction on in situ chest drain? Interact Cardiovasc Thorac Surg 2014;18:825-9. [Crossref] [PubMed]
Cite this article as: Nyi T, Chrastek D, Shah S, Kouritas V. Tracheal laceration causing important post-intubation delayed subcutaneous emphysema and ventilatory deterioration in a COVID-19 patient with severe rheumatoid arthritis: a case report. Mediastinum 2021;5:30.