
The role of immunotherapy for management of advanced thymic epithelial tumors: a narrative review
Introduction
The advent of immunotherapy is rapidly changing the treatment paradigm for various cancers and improving patient outcomes. Underlying these advances is a clearer understanding of the complex relationship between cancer and the immune system. The development of cancer is fundamentally a disorder of immune surveillance and an inability to eliminate neoplastic cells (1). Anti-cancer immunity is influenced by features of the tumor and tumor microenvironment, such as tumor-associated antigen and major histocompatibility complex (MHC) antigen expression, cytokine levels, relative proportions of immune stimulatory and immunosuppressive cells, and the status of immune checkpoints, as well as host and environmental factors (2).
Based on this knowledge, a number of immunotherapeutic interventions have been developed for treatment of advanced cancers including vaccines, adoptive T-cell therapy and immune checkpoint blockade (3). Antibodies targeting the immunosuppressive checkpoints, programed death-1 (PD-1) or its ligand (PD-L1) are now an integral component of cancer therapy and are being widely evaluated in combination with other treatment modalities, including chemotherapy, radiation therapy and other forms of immunotherapy (4). Emerging data provide proof of long-term safety and durable benefit of immunotherapy with an improvement in survival in patients with advanced cancers (5,6).
In this paper we review the role of immunotherapy for management of advanced thymic epithelial tumors (TETs) and discuss the impact of unique aspects of TET biology on the risks and potential benefits of immune modulation. We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/med-20-62).
Data sources
In order to select data for inclusion in this narrative review, we performed a literature search using the National Library of Medicine’s PubMed® database. Selection of papers was limited to the English language but there were no limits on the type of report or the time of publication.
TETs and the immune system
The thymus gland plays a key role in development of normal immunological function including the body’s ability to recognize its own tissue and develop immunological self-tolerance (7,8). This process involves the migration of lymphoid progenitor cells into the thymus followed by a series of steps that culminates in the formation of CD4+CD8+ double positive cells that express a functional T cell receptor (TCR). Subsequently, a subset of T cells expressing either CD4 or CD8 enter the thymic medulla and are exposed to organ-specific antigens. Autoreactive T cells undergo apoptosis and the remainder enter the peripheral circulation (9). Medullary thymic epithelial cell expression of tissue-restricted antigens is controlled by the transcription factors, AIRE (autoimmune regulator) and Fezf2 (8,10,11). A breakdown in immunological tolerance results in entry of autoreactive T cells into the peripheral circulation and increases the risk for development of autoimmunity and immunodeficiency.
The association between TETs, especially thymomas, and paraneoplastic autoimmunity is well recognized (12). Defective immune tolerance in patients with TETs is attributed to multiple factors including decreased expression of AIRE and Fezf2, altered thymic architecture and downregulation of MHC class 2 antigens (12).
An additional mechanism for development of paraneoplastic autoimmunity in patients with TETs appears to rely on structural similarities between antigens overexpressed by neoplastic cells and autoantigens expressed on target organs by the process of molecular mimicry. Data from The Cancer Genome Atlas program for thymomas reveals overexpression of the mid-sized neurofilament gene (NEF) in thymomas associated with myasthenia gravis (MG), which shares sequences coding for acetylcholine receptor (AChR) and titin epitopes that are associated with MG (13).
In addition to autoimmune paraneoplastic disease, T-cell dysfunction in patients with TETs can also manifest clinically in the form of an immunodeficiency state. Acquired T-cell deficiency can be accompanied by hypogammaglobulinemia and increase the risk of opportunistic infections (14).
Determinants of response to immune checkpoint inhibitors (ICIs)
Despite the widespread use of ICIs for cancer therapy, a large proportion of patients with advanced cancers do not derive significant clinical benefit. This observation has spurred the development of predictive biomarkers to help identify patients most likely to benefit from treatment (15).
Tumor cell PD-L1 expression and tumor mutation burden (TMB) have been widely evaluated as biomarkers of response to ICIs (16). Tumors with high PD-L1 expression and/or high TMB are more likely to respond to ICI therapy (17). However, the predictive value of these biomarkers is not uniform across tumor types (15).
Microsatellite instability caused by defects in DNA mismatch repair (MMR) results in a high TMB and an increase in tumor-infiltrating lymphocytes (18). Consequently, tumors with MMR deficiency are more likely to respond to immune checkpoint blockade, as has been observed in clinical trials (19). Higher response rates and longer survival following treatment with ICIs has also been seen in patients with concurrent mutations in genes involved in other DNA damage repair pathways, such as base excision repair and homologous recombination (20).
An 18-gene T-cell inflamed gene expression profile (GEP) that reflects a T-cell activated tumor microenvironment has been evaluated as a potential biomarker of response to ICIs and is shown to be associated with response and an improvement in survival with pembrolizumab across various tumor types (17).
To improve selection of patients for immunotherapy, several novel predictive biomarkers are under active investigation. These include somatic mutations, HLA diversity, cytokines such as interleukin-6, TCR clonality and host factors such as the gut microbiome (15,21).
Rationale for use of ICIs for treatment of TETs
PD-L1 expression is common across the histological spectrum of TETs with higher expression observed more frequently in clinically aggressive histological subgroups (22,23). However, TETs have a low TMB and microsatellite instability is extremely rare (13,24,25). Despite a low TMB, alterations in genes involved in DNA repair such as BRCA2, BAP1 and ATM have been observed in up to 13% of recurrent TETs (26,27). It is unclear if these genomic alterations increase the likelihood of response to immune checkpoint blockade.
In order to establish a rationale for use of immunotherapy in patients with recurrent TETs, recent research has focused on the unique biology of thymoma and thymic carcinoma. Functional analysis of CD4 and CD8 single-positive T cells by flow cytometry has revealed an increased proportion of Tim-3- and CD103-expressing T cells in type B3 thymoma and thymic carcinoma. Cytokine production and cytotoxicity of effector T cells was enhanced to a greater degree by the PD-1 inhibitor, nivolumab in these histological subtypes (28). Preclinical studies have also shown significant enhancement of antitumor activity of ICIs in the presence of Aire-deficiency, which is commonly observed in thymomas (29).
These observations, coupled with tumor cell PD-L1 expression, provide a rationale for evaluation of ICIs in TETs despite low TMB and absence of microsatellite instability.
Immunotherapy for TETs
The safety and clinical activity of immunotherapy for TETs has been reported in five completed prospective trials, including four studies that have evaluated PD-1/PD-L1-directed monoclonal antibodies and one trial that evaluated a WT-1-based peptide vaccine.
Clinical activity
Between December 2013 to October 2014, 7 patients with recurrent thymoma and 1 patient with recurrent thymic carcinoma were enrolled in a phase 1 dose-escalation study of the anti-PD-L1 antibody, avelumab (NCT01772004) and treated at two dose levels (10 or 20 mg/kg) (30). Two (29%) patients had a confirmed partial response and 5 (63%) patients had stable disease, including 2 individuals who met response evaluation criteria in solid tumors (RECIST) for an unconfirmed partial response. Two responses occurred at each dose level tested and 3 of 4 responses occurred after administration of a single dose of avelumab. The duration of response ranged from 4 to 17 weeks, despite discontinuation of treatment after a single dose in 3 of 4 patients. These results provided initial evidence of anti-tumor activity of PD-L1-directed therapy in relapsed thymoma.
From March 2015 to December 2016, 41 patients with relapsed thymic carcinoma were enrolled in a single-arm phase 2 trial of pembrolizumab (anti-PD-1 antibody; NCT02364076) and received 200 mg intravenously every 3 weeks for up to 2 years (31). The overall response rate (ORR) was 22.5%. The median duration of response (DOR) was 22.4 months, median progression free survival (PFS) was 4.2 months and median overall survival (OS) was 24.9 months. The clinical activity of pembrolizumab in recurrent TETs was confirmed in a subsequent phase 2 trial that enrolled 26 patients with relapsed thymic carcinoma and 7 patients with relapsed thymoma (NCT02607631) (32). The ORR was 28.6% in patients with thymoma and 19.2% in patients with thymic carcinoma. The median DOR was not reached in patients with thymoma and it was 9.7 months in patients with thymic carcinoma. Median PFS was 6.1 months for both groups. Median OS was not reached for patients with thymoma and it was 14.5 months for the thymic carcinoma cohort.
Nivolumab, an anti-PD-1 antibody, has been evaluated in patients with recurrent thymic carcinoma in a single-arm phase 2 clinical trial (PRIMER study) (33). Fifteen patients were enrolled and received nivolumab 3 mg/kg intravenously every 2 weeks. No objective responses were observed in this study. The median PFS was 3.8 months and median OS was 14.1 months.
Oji and colleagues have evaluated a novel immunotherapeutic intervention with a WT1 peptide vaccine in patients with advanced TETs (34). Fifteen patients (4 thymoma, 11 thymic carcinoma) were enrolled in a phase 2 trial and received a 9 mer-WT1-derived peptide vaccine intradermally once a week. After completion of a planned study period of 3 months, treatment was continued at 2-4-week intervals until disease progression or development of intolerable adverse events. Although no objective responses were observed in this study, 75% of patients achieved disease stabilization and a majority of patients demonstrated a WT1-specific immune response. The median duration of treatment was 133 days in patients with recurrent thymic carcinoma and 683 days in patients with recurrent thymoma.
These observations provide evidence of the clinical activity of immunotherapy in patients with relapsed TETs and are summarized in Table 1. However, clinical benefit is not uniform and highlights the need to develop biomarkers to identify patients most likely to benefit from treatment.
Table 1
| Treatment | Number of patients | Response rate (%) | Median PFS (months) | Median OS (months) |
|---|---|---|---|---|
| Pembrolizumab ( |
||||
| Thymoma | 7 | 28.6 | 6.1 | Not reached |
| Thymic carcinoma | 26 | 19.2 | 6.1 | 14.5 |
| Pembrolizumab ( |
||||
| Thymic carcinoma | 40 | 22.5 | 4.2 | 24.9 |
| Avelumab ( |
||||
| Thymoma | 7 | 28.5 | NR | NR |
| Nivolumab ( |
||||
| Thymic carcinoma | 15 | 0 | 3.8 | 14.1 |
| WT1 peptide vaccine ( |
||||
| Thymoma | 4 | 0 | NR | NR |
| Thymic carcinoma | 11 | 0 | NR | NR |
PFS, progression-free survival; OS, overall survival; NR, not reported.
Biomarkers for response to immunotherapy
Patients enrolled in 3 of the 5 clinical trials described above experienced an objective response to treatment. In the trials evaluating pembrolizumab, high PD-L1 expression (defined as > 50% tumor cell PD-L1 staining by immunohistochemistry) was associated with higher response rates and longer survival (31,32). However, it should be noted that the majority of patients enrolled in these trials had thymic carcinoma. It is unclear if PD-L1 expression is equally effective in predicting response and survival in patients with advanced thymoma.
T-cell gene expression profiling was conducted in the study evaluating pembrolizumab in thymic carcinoma (NCT02364076) and revealed higher expression of the 18-gene T-cell-inflamed interferon-g gene expression profile among responders (31). There was no correlation between somatic mutations detected by targeted exome sequencing and response to treatment (31).
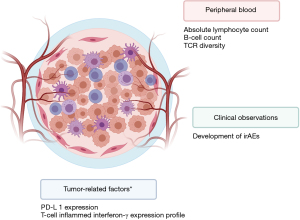
In the phase I trial of avelumab, responders had a higher pre-treatment absolute lymphocyte count, lower frequencies of B cells, regulatory T cells, conventional dendritic cells, and natural killer cells and higher baseline TCR diversity (30). All responders also developed immune-related adverse events (irAEs). These observations are illustrated in Figure 1.
Safety
One of the biggest challenges associated with the use of immunotherapy for TETs is the risk for development of potentially life-threatening immune-mediated toxicity.
Results from prospective clinical trials and several case reports confirm the increased risk of irAEs and highlight the predisposition toward muscle and neuromuscular toxicity (30-36). Polymyositis (all grades) has been observed in 8% to 57% of TET patients treated with ICIs. Corresponding figures for myocarditis and myasthenia gravis are 5% to 57% and 3% to 14%, respectively, with a higher incidence in patients with advanced thymoma (30-32). Musculoskeletal and neuromuscular irAEs generally occur early and have been observed within 1–6 weeks of initiation of ICI therapy (30-32). It should be noted that the elevated risk of immune toxicity is present across the histological spectrum of TETs and can be observed in individuals with thymic carcinoma as well (31,32,36). Furthermore, muscle and neuromuscular toxicity in TET patients is not limited to ICI therapy alone and has been observed in patients receiving a cancer vaccine. One of 4 patients with thymoma receiving a WT1 peptide vaccine developed myasthenia gravis 26 months after initiation of treatment (34). Compared with observations of neuromuscular complications in patients with TETs receiving ICIs, the delayed onset of myasthenia gravis in this case highlights the need for close monitoring for immune toxicity throughout the course of treatment.
In contrast, neuromuscular and cardiac toxicity is uncommon in patients with non-thymic cancers receiving ICIs. The incidence of ICI-induced neurological adverse events is 1% or less in large studies (37). Myopathies are observed in less than 1% of patients receiving PD-1-directed anticancer therapy, whereas myocarditis has been reported in 0.4% to 1% of patients receiving ICIs (38-40).
Additionally, with the exception of myasthenia gravis, neuromuscular disorders are uncommon manifestations of paraneoplastic autoimmunity in patients with TETs. The prevalence of polymyositis is 1% to 5% and paraneoplastic myocarditis is observed in fewer than 1% of patients with thymoma (12).
TET patients receiving ICIs have also been observed to develop other relatively uncommon irAEs such as type 1 diabetes mellitus, sicca syndrome and acquired coagulopathy (Figure 2) (31,41,42). Table 2 lists irAEs reported in published clinical trials of immunotherapy in relapsed TETs.
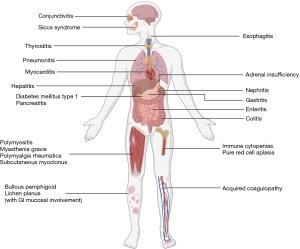
Table 2
| irAE, n [%] | Pembrolizumab ( |
Pembrolizumab ( |
Avelumab ( |
Nivolumab ( |
WT1 peptide vaccine ( |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymoma (n=7) | Thymic ca (n=26) | Thymic ca (n=40) | Thymoma (n=7) | Thymic ca (n=15) | Thymoma (n=4) | Thymic ca (n=11) | |||||
| Polymyositis | 0 | 0 | 3 [8] | 4 [57] | 3 [20] | 0 | 0 | ||||
| Myocarditis | 3 [43] | 0 | 2 [5] | 4 [57] | 0 | 0 | 0 | ||||
| Myasthenia gravis | 1 [14] | 2 [8] | 1 [3] | 0 | 0 | 1 [25] | 0 | ||||
| Subacute myoclonus | 0 | 1 [4] | 0 | 0 | 0 | 0 | 0 | ||||
| Cranial neuropathy | 0 | 0 | 0 | 1 [14] | 0 | 0 | 0 | ||||
| Conjunctivitis | 1 [14] | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Pneumonitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Enteritis | 0 | 0 | 0 | 1 [14] | 0 | 0 | 0 | ||||
| Colitis† | 1 [14] | 0 | 0 | 0 | 3 [20] | 0 | 0 | ||||
| Hepatitis | 2 [29] | 2 [8] | 5 [13] | 4 [57] | 11 [73]‡ | 0 | 0 | ||||
| Pancreatitis | 0 | 0 | 1 [3] | 0 | 0 | 0 | 0 | ||||
| Nephritis# | 1 [14] | 0 | 0 | 0 | 2 [13] | 0 | 0 | ||||
| Thyroiditis* | 2 [29] | 1 [4] | 0 | 0 | 1 [7] | 0 | 0 | ||||
| Adrenal insufficiency | 0 | 0 | 0 | 0 | 1 [7] | 0 | 0 | ||||
| Bullous pemphigoid | 0 | 0 | 1 [3] | 0 | 0 | 0 | 0 | ||||
| Other skin conditions^ | 2 [29] | 5 [19] | 0 | 0 | 4 [27] | 0 | 0 | ||||
| Pure red cell aplasia | 0 | 0 | 0 | 0 | 0 | 1 [25] | 0 | ||||
†, includes three cases described using the term “diarrhea”; ‡, represents eight cases with aspartate transaminase elevation (AST) of any grade and three cases with alanine transaminase (ALT) elevation of any grade (etiology not defined and unclear if three subjects had concurrent elevation of AST and ALT); #, includes one case described using the term “creatinine increased”; *, includes one case of hypothyroidism; ^includes 11 cases described using the terms “dermatitis”, “skin rash” and “pruritis”. irAE, immune-related adverse event; ca, carcinoma.
Taken together, these observations are indicative of an increased risk of immune-mediated toxicity in patients with TETs receiving immunotherapy, regardless of the treatment modality and histological characteristics of the tumor and stress the need for extreme caution while using immunotherapy for treatment of TETs.
Biomarkers for toxicity
The reasons for an increased incidence of irAEs in patients with TETs receiving immunotherapy are incompletely understood, although it appears to be fundamentally related to defective immune self-tolerance and persistence of autoreactive T-cells.
Clinical trials of avelumab and pembrolizumab provide early evidence of potential biomarkers of toxicity to immunotherapy in patients with thymoma and thymic carcinoma with no clinical history of paraneoplastic autoimmunity.
In two trials evaluating pembrolizumab in relapsed TETs, there was no correlation between development of irAEs and the degree of PD-L1 expression or the presence of somatic mutations detected by targeted exome sequencing (31,32).
However, patients with relapsed thymoma who developed immune-related myositis following treatment with avelumab were found to have detectable titers of AChR-binding autoantibodies prior to start of treatment, whereas patients who did not develop myositis had no pre-treatment AChR autoantibodies (43). In addition, patients who developed irAEs also had profound B-cell cytopenia and lower levels of regulatory T cells and conventional dendritic cells (30,43). Unsupervised hierarchical clustering of major peripheral blood mononuclear cell (PBMC) subsets prior to treatment revealed an immune phenotype that was associated with development of autoimmunity and TCR diversity in pretreatment PBMCs was higher in patients experiencing irAEs (30).
If validated in larger studies, these observations can help select patients with thymoma and thymic carcinoma for immunotherapy who are at lower risk for development of irAEs.
Mitigation of immune toxicity
Although careful consideration of clinical history and the development of predictive biomarkers might potentially lessen the risk of irAEs in patients with thymoma and thymic carcinoma, it is unlikely that these risks can be eliminated due to the underlying biology of these diseases. Hence, it is important to develop other risk mitigation strategies such as prophylactic use of immunosuppressants in conjunction with immunotherapy and strategies to rechallenge patients who have experienced immune toxicity previously (44).
There is a growing body of data on the concurrent use of immunosuppression with immunotherapy. In a small series of five patients with solid tumors who developed immune-related enterocolitis upon treatment with ICIs, either as monotherapy or in combination, concurrent treatment with the tumor necrosis factor (TNF)-α inhibitor, infliximab and ICIs was found to be safe and prevent further episodes of immune-related enterocolitis (45). A similar approach using cyclosporine A to prevent muscle-related immune toxicity in patients with relapsed TETs is under investigation in an ongoing phase II trial of avelumab (NCT03076554). The effect of concurrent immunosuppression on the anti-tumor activity of ICIs remains to be determined.
The safety of resuming immunotherapy after resolution of an initial episode of immune-mediated toxicity has been evaluated in a few recently reported studies. No further episodes of irAEs were observed upon resumption of ICIs in 45% to 66% of patients evaluated in these studies indicating the feasibility of rechallenging patients with immunotherapy after resolution of irAEs (46-49). However, it should be stressed that the decision to resume immunotherapy should be based on the nature and severity of the initial episode of immune toxicity and is generally not recommended for patients who develop severe or life-threatening irAEs, such as myocarditis.
Future directions
ICIs have been largely successful in eliciting durable responses and improving survival of patients with several advanced cancers, including relapsed TETs. Clinical trials are currently underway to evaluate combinations of ICIs with other systemic therapies, including targeted therapies and other forms of immunotherapy in an effort to improve clinical outcomes (50). Ongoing trials evaluating immunotherapy combinations in patients with thymic cancers are listed in Table 3.
Table 3
| Intervention | Target | Phase | Histology | Primary objective | Clinical trial ID |
|---|---|---|---|---|---|
|
|
|||||
| Avelumab + Axitinib (CAVEATT trial) | PD-L1, VEGFR, PDGFR | II | B3 thymoma, Thymic carcinoma | Response rate | 2017-004048-38 |
| Nivolumab + Vorolanib | PD-1, VEGFR, PDGFR | I/II | Thymic carcinoma* | Phase I: Safety and tolerability, Phase II: Response rate | NCT03583086 |
| Pembrolizumab + Sunitinib | PD-1, VEGFR2, PDGFR-β, c-kit, FLT3 | II | Thymic carcinoma | Response rate | NCT03463460 |
|
|
|||||
| Nivolumab + Ipilimumab (NIVOTHYM trial) | PD-1, CTLA-4 | II | B3 thymoma, thymic carcinoma | 6-month PFS | NCT03134118 |
| Bintrafusp alfa | PD-L1, TGF-βRII | II | Thymoma (all subtypes), Thymic carcinoma | Response rate | NCT04417660 |
| Pembrolizumab + Epacadostat | PD-1, IDO1 | II | Thymic carcinoma | Response rate | NCT02364076 |
*, Patients with refractory thoracic cancers, including thymic carcinoma are eligible for this trial. PD-L1, programed death ligand-1; PD-1, programed death-1; VEGFR, vascular endothelial growth factor receptors; PDGFR, platelet-derived growth factor receptors; FLT3, Fms-related tyrosine kinase 3; CTLA-4, cytotoxic T-lymphocyte-associated antigen 4; TGF-βRII, transforming growth factor beta receptor type II; IDO1, indoleamine 2,3-dioxygenase; PFS, progression-free survival.
Current research is also focused on identifying biomarkers of response and toxicity to ICIs (15,21). In patients with thymoma, the presence of detectable AChR-binding autoantibodies, B-cell cytopenia and high TCR diversity at baseline appear to be associated with an increased risk for development of immune toxicities (30,43). Validation of these results in larger clinical trials and evaluation of novel biomarkers such as the tumor mutational profile and the gut microbiome holds the promise of improving the safety profile of immunotherapy for thymic cancers and identifying patients most likely to benefit from treatment.
Other clinical questions that warrant further investigation include the ability to use immunotherapy for treatment of TET patients with associated autoimmune paraneoplastic diseases and the feasibility of rechallenging TET patients with immunotherapy after resolution of a previous episode of immune-mediated toxicity. There is also a pressing need to evaluate the use of immunosuppressive drugs concurrently with immunotherapy for primary or secondary prophylaxis of irAEs in future clinical trials.
Conclusions
The clinical activity of ICIs in patients with relapsed thymoma and thymic carcinoma has been demonstrated in prospective clinical trials with several patients achieving durable responses. Immunotherapy also appears to improve survival compared with currently available treatments for patients with recurrent disease. However, treatment can be associated with the development of severe and life-threatening immune toxicity, especially in patients with thymoma. Hence, we recommend consideration of immunotherapy for treatment of TETs in the context of clinical trials with close monitoring for and aggressive management of irAEs. Ongoing advances in biomarker research increase the likelihood of identifying patients likely to benefit from treatment with a reduced risk of developing severe irAEs. Several unanswered questions related to clinical management of immune-mediated toxicity are being addressed in ongoing clinical trials and hold the promise of making the use of immunotherapy feasible and safe for patients with recurrent thymoma and thymic carcinoma.
Acknowledgments
Figures were created with BioRender.com.
Funding: This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mirella Marino and Brett W. Carter) for the series “Dedicated to the 10th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2019)” published in Mediastinum. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/med-20-62
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-62). The series “Dedicated to the 10th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2019)” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565-70. [Crossref] [PubMed]
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541:321-30. [Crossref] [PubMed]
- Martin SD, Coukos G, Holt RA, et al. Targeting the undruggable: immunotherapy meets personalized oncology in the genomic era. Ann Oncol 2015;26:2367-74. [Crossref] [PubMed]
- Wilky BA. Immune checkpoint inhibitors: The linchpins of modern immunotherapy. Immunol Rev 2019;290:6-23. [Crossref] [PubMed]
- Garon EB, Hellmann MD, Rizvi NA, et al. Five-Year Overall Survival for Patients With Advanced NonSmall-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. J Clin Oncol 2019;37:2518-2527. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated With Nivolumab. JAMA Oncol 2019;5:1411-20. [Crossref] [PubMed]
- Miller JFAP. The function of the thymus and its impact on modern medicine. Science 2020;369:eaba2429 [Crossref] [PubMed]
- Cheng M, Anderson MS. Thymic tolerance as a key brake on autoimmunity. Nat Immunol 2018;19:659-64. [Crossref] [PubMed]
- Maverakis E, Goodarzi H, Wehrli LN, et al. The etiology of paraneoplastic autoimmunity. Clin Rev Allergy Immunol 2012;42:135-44. [Crossref] [PubMed]
- Anderson MS, Venanzi ES, Klein L, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science 2002;298:1395-401. [Crossref] [PubMed]
- Takaba H, Morishita Y, Tomofuji Y, et al. Fezf2 Orchestrates a Thymic Program of Self-Antigen Expression for Immune Tolerance. Cell 2015;163:975-87. [Crossref] [PubMed]
- Lippner EA, Lewis DB, Robinson WH, et al. Paraneoplastic and Therapy-Related Immune Complications in Thymic Malignancies. Curr Treat Options Oncol 2019;20:62. [Crossref] [PubMed]
- Radovich M, Pickering CR, Felau I, et al. The Integrated Genomic Landscape of Thymic Epithelial Tumors. Cancer Cell 2018;33:244-58.e10. [Crossref] [PubMed]
- Christopoulos P, Fisch P. Acquired T-Cell Immunodeficiency in Thymoma Patients. Crit Rev Immunol 2016;36:315-27. [Crossref] [PubMed]
- Bai R, Lv Z, Xu D, et al. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark Res 2020;8:34. [Crossref] [PubMed]
- Huang RSP, Haberberger J, Severson E, et al. A pan-cancer analysis of PD-L1 immunohistochemistry and gene amplification, tumor mutation burden and microsatellite instability in 48,782 cases. Mod Pathol 2021;34:252-63. [Crossref] [PubMed]
- Ott PA, Bang YJ, Piha-Paul SA, et al. T-Cell-Inflamed Gene-Expression Profile, Programmed Death Ligand 1 Expression, and Tumor Mutational Burden Predict Efficacy in Patients Treated With Pembrolizumab Across 20 Cancers: KEYNOTE-028. J Clin Oncol 2019;37:318-27. [Crossref] [PubMed]
- Zhang Y, Sun Z, Mao X, et al. Impact of mismatch-repair deficiency on the colorectal cancer immune microenvironment. Oncotarget 2017;8:85526-36. [Crossref] [PubMed]
- Marcus L, Lemery SJ, Keegan P, et al. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin Cancer Res 2019;25:3753-8. [Crossref] [PubMed]
- Wang Z, Zhao J, Wang G, et al. Comutations in DNA Damage Response Pathways Serve as Potential Biomarkers for Immune Checkpoint Blockade. Cancer Res 2018;78:6486-96. [Crossref] [PubMed]
- Burdett N, Desai J. New biomarkers for checkpoint inhibitor therapy. ESMO Open 2020;5:e000597 [Crossref] [PubMed]
- Song JS, Kim D, Kwon JH, et al. Clinicopathologic Significance and Immunogenomic Analysis of Programmed Death-Ligand 1 (PD-L1) and Programmed Death 1 (PD-1) Expression in Thymic Epithelial Tumors. Front Oncol 2019;9:1055. [Crossref] [PubMed]
- Sekine I, Aida Y, Suzuki HJM. Expression patterns and prognostic value of programmed death ligand-1 and programmed death 1 in thymoma and thymic carcinoma. Mediastinum 2018;2:54. [Crossref]
- Vanderwalde A, Spetzler D, Xiao N, et al. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med 2018;7:746-56. [Crossref] [PubMed]
- Zhou R, Zettl A, Strobel P, et al. Thymic epithelial tumors can develop along two different pathogenetic pathways. Am J Pathol 2001;159:1853-60. [Crossref] [PubMed]
- Wang Y, Thomas A, Lau C, et al. Mutations of epigenetic regulatory genes are common in thymic carcinomas. Sci Rep 2014;4:7336. [Crossref] [PubMed]
- Enkner F, Pichlhofer B, Zaharie AT, et al. Molecular Profiling of Thymoma and Thymic Carcinoma: Genetic Differences and Potential Novel Therapeutic Targets. Pathol Oncol Res 2017;23:551-64. [Crossref] [PubMed]
- Yamamoto Y, Iwahori K, Funaki S, et al. Immunotherapeutic potential of CD4 and CD8 single-positive T cells in thymic epithelial tumors. Sci Rep 2020;10:4064. [Crossref] [PubMed]
- Benitez AA, Khalil-Aguero S, Nandakumar A, et al. Absence of central tolerance in Aire-deficient mice synergizes with immune-checkpoint inhibition to enhance antitumor responses. Commun Biol 2020;3:355. [Crossref] [PubMed]
- Rajan A, Heery CR, Thomas A, et al. Efficacy and tolerability of anti-programmed death-ligand 1 (PD-L1) antibody (Avelumab) treatment in advanced thymoma. J Immunother Cancer 2019;7:269. [Crossref] [PubMed]
- Giaccone G, Kim C, Thompson J, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol 2018;19:347-55. [Crossref] [PubMed]
- Cho J, Kim HS, Ku BM, et al. Pembrolizumab for Patients With Refractory or Relapsed Thymic Epithelial Tumor: An Open-Label Phase II Trial. J Clin Oncol 2019;37:2162-70. [Crossref] [PubMed]
- Katsuya Y, Horinouchi H, Seto T, et al. Single-arm, multicentre, phase II trial of nivolumab for unresectable or recurrent thymic carcinoma: PRIMER study. Eur J Cancer 2019;113:78-86. [Crossref] [PubMed]
- Oji Y, Inoue M, Takeda Y, et al. WT1 peptide-based immunotherapy for advanced thymic epithelial malignancies. Int J Cancer 2018;142:2375-82. [Crossref] [PubMed]
- Chen Q, Huang DS, Zhang LW, et al. Fatal myocarditis and rhabdomyolysis induced by nivolumab during the treatment of type B3 thymoma. Clin Toxicol (Phila) 2018;56:667-71. [Crossref] [PubMed]
- Szuchan C, Elson L, Alley E, et al. Checkpoint inhibitor-induced myocarditis and myasthenia gravis in a recurrent/metastatic thymic carcinoma patient: a case report. Eur Heart J Case Rep 2020;4:1-8. [Crossref] [PubMed]
- Möhn N, Beutel G, Gutzmer R, et al. Neurological Immune Related Adverse Events Associated with Nivolumab, Ipilimumab, and Pembrolizumab Therapy-Review of the Literature and Future Outlook. J Clin Med 2019;8:1777. [Crossref] [PubMed]
- Liewluck T, Kao JC, Mauermann ML. PD-1 Inhibitor-associated Myopathies: Emerging Immune-mediated Myopathies. J Immunother 2018;41:208-11. [Crossref] [PubMed]
- Salem JE, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol 2018;19:1579-89. [Crossref] [PubMed]
- Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol 2018;71:1755-64. [Crossref] [PubMed]
- Warner BM, Baer AN, Lipson EJ, et al. Sicca Syndrome Associated with Immune Checkpoint Inhibitor Therapy. Oncologist 2019;24:1259-69. [Crossref] [PubMed]
- Joseph JJ, Rajan A, Gulley JL, et al. Acquired Coagulopathy With Immune Checkpoint Inhibitors: An Underrecognized Association Between Inflammation and Coagulation. JTO Clin Res Rep 2020;1:100049
- Mammen AL, Rajan A, Pak K, et al. Pre-existing antiacetylcholine receptor autoantibodies and B cell lymphopaenia are associated with the development of myositis in patients with thymoma treated with avelumab, an immune checkpoint inhibitor targeting programmed death-ligand 1. Ann Rheum Dis 2019;78:150-2. [Crossref] [PubMed]
- Haanen J, Ernstoff M, Wang Y, et al. Rechallenge patients with immune checkpoint inhibitors following severe immune-related adverse events: review of the literature and suggested prophylactic strategy. J Immunother Cancer 2020;8:e000604 [Crossref] [PubMed]
- Badran YR, Cohen JV, Brastianos PK, et al. Concurrent therapy with immune checkpoint inhibitors and TNFalpha blockade in patients with gastrointestinal immune-related adverse events. J Immunother Cancer 2019;7:226. [Crossref] [PubMed]
- Santini FC, Rizvi H, Plodkowski AJ, et al. Safety and Efficacy of Re-treating with Immunotherapy after Immune-Related Adverse Events in Patients with NSCLC. Cancer Immunol Res 2018;6:1093-9. [Crossref] [PubMed]
- Pollack MH, Betof A, Dearden H, et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol 2018;29:250-5. [Crossref] [PubMed]
- Simonaggio A, Michot JM, Voisin AL, et al. Evaluation of Readministration of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients With Cancer. JAMA Oncol 2019;5:1310-7. [Crossref] [PubMed]
- Abu-Sbeih H, Ali FS, Naqash AR, et al. Resumption of Immune Checkpoint Inhibitor Therapy After Immune-Mediated Colitis. J Clin Oncol 2019;37:2738-45. [Crossref] [PubMed]
- Kon E, Benhar I. Immune checkpoint inhibitor combinations: Current efforts and important aspects for success. Drug Resist Updat 2019;45:13-29. [Crossref] [PubMed]
Cite this article as: Rajan A, Mullenix C, Shelat M, Zhao C. The role of immunotherapy for management of advanced thymic epithelial tumors: a narrative review. Mediastinum 2021;5:23.


