
Diagnostic accuracy of endobronchial ultrasound-transbronchial needle aspiration (EBUS-TBNA) for mediastinal lymph node staging of lung cancer
Introduction
Lung cancer is one of the leading causes of cancer-related mortality around the world. Diagnosis and accurate staging are of the essence in order to establish the appropriate treatment plan. Mediastinal lymph nodes involvement is the most important parameter to define the therapeutic path, and particularly to decide whether a patient can be offered a potentially curative surgery. Endobronchial ultrasound-transbronchial needle aspiration (EBUS-TBNA), together with oesophageal ultrasound (EUS), is considered the preferred approach for mediastinal lymph node assessment in non-small cell lung cancer (NSCLC) (1), and is useful in case of centrally-located tumours too. In fact, compared to mediastinoscopy, EBUS-TBNA showed excellent diagnostic performances (2), and it also allows to sample hilar and intrapulmonary lymph nodes. Moreover, EBUS-TBNA is less invasive, and is usually performed in an outpatient setting or with a brief post-procedure hospitalization, using topical anaesthesia and mild sedation. On the other hand, EBUS-TBNA can only access nodes in the anterosuperior mediastinum, sometimes requiring to be combined with EUS in order to sample the other nodes in the mediastinum; the two procedures can be performed sequentially in the same session. Furthermore, the accuracy of EBUS-TBNA depends on the endoscopist’s skills (3). In this scenario, we aimed to review the current evidence on EBUS-TBNA diagnostic accuracy and report the experience in our centre.
Indications and contraindications
EBUS-TBNA represents the gold-standard procedure for diagnosis and especially for staging of lung cancer: it allows to evaluate the neoplastic involvement of mediastinal and hilar lymph nodes sampled. This procedure has also a crucial role in detecting nodal localizations of metastasis, other primary cancers, lymphoma, infectious and granulomatous diseases (i.e., sarcoidosis). EBUS-TBNA also plays an important role in mediastinal restaging after induction therapy for locally advanced stage III NSCLC (4). Since those patients who successfully respond to neoadjuvant treatment can be candidates for curative resection, mediastinal N2 lymph node previously proven to be positive, should preferably be re-biopsied. Moreover, in case of endobronchial lesions, EBUS-TBNA allows to evaluate the extension of airways invasion for surgical planning (for example in case of sleeve pulmonary resections), and to discern between airway invasion and tumour compression (5). The role of EBUS-TBNA in the diagnosis of lymphoproliferative disorders, instead, is still controversial, as treatment regimens depend on the specific subtype and histologic grade, and a cytologic sample may not always be adequate to obtain such information. Many studies reported a heterogeneous and often lower diagnostic accuracy for EBUS-TBNA for lymphoma, compared to other neoplasm (6). The CHEST guidelines published in 2016 suggested, in an ungraded consensus-based statement, that this technique is acceptable in case of suspected lymphoma, as an initial minimally-invasive diagnostic test (7), and to obtain a differential diagnosis between lymphoid and epithelial neoplasms. Contraindications to EBUS-TBNA are the same as those of bronchoscopy, such as: severe respiratory (acute exacerbation of chronic obstructive pulmonary disease or asthma) and cardiologic comorbidities (recent myocardial infarction, poorly controlled heart failure, haemodynamic instability, arrythmias), and coagulopathies (both congenital and drug-induced). Antiplatelet and anticoagulation therapy should always be held in order to reduce the risk of post-operative bleeding (3).
Diagnostic accuracy of EBUS-TBNA: current evidence
Despite mediastinoscopy having been considered the best option for some time, to this day EBUS-TBNA represents the first choice for invasive mediastinal staging, according to international recommendations from American College of Chest Physicians (ACCP) (7-9), European Society of Thoracic Surgeons (ESTS) (4), National Comprehensive Cancer Network (NCCN) (10) and European Society for Medical Oncology (ESMO) (11). EBUS-TBNA provides a valid minimally invasive alternative to mediastinoscopy and anterior mediastinotomy in reason of equivalent diagnostic accuracy, more excellent safety, and lower total medical costs.
Since the amount of radiological false negatives is not irrelevant, current European guidelines recommend invasive mediastinal staging in patients with central tumours despite positron emission tomography (PET)-negative mediastinal lymph nodes, clinical N1 disease, enlarged (short axis >1 cm) mediastinal lymph nodes on computed tomography (CT)-scan, or tumours larger than 3 cm. Video-assisted mediastinoscopy (VAM) is recommended in case of negative result by endosonographic evaluation (4,11).
EBUS-TBNA seems to have high specificity and good sensitivity in pathological results; these findings are influenced by lymph nodes dimensions, station level (higher for stations 4L and 7) and operator experience.
Ernst et al. in a prospective crossover study, demonstrated higher diagnostic accuracy (91%) of EBUS-TBNA compared to mediastinoscopy (78%) (P=0.007), with major difference in diagnostic yield when considering station 7 (98% for EBUS-TBNA vs. 78% for mediastinoscopy, P=0.007) (12). Um et al. (13) confirmed these results in a study which showed inferior diagnostic yield using mediastinoscopy compared to EBUS-TBNA on station 7 (52.4% vs. 81%, P=0.027) and 4L lymph nodes (75% vs. 82.5%). Murthi et al. retrospectively analysed patients who underwent EBUS-TBNA of mediastinal and hilar lymph nodes followed by surgical intervention (mediastinoscopy and/or lymph node dissection). They found a 55.1% sensitivity (95% CI: 41.5–68.3%), 100% specificity, 100% positive predictive value (PPV), 75.7% negative predictive value (NPV) (95% CI: 70–80.5%) and 81.2% accuracy. Disease-specific accuracy was 81.7%, 84% and 78.9% for the diagnosis of cancer, NSCLC and non-cancer lesions, respectively. The authors also found a relatively high number of lymph nodes that were involved in pathology but were not been sampled by EBUS-TBNA, leading to a false-negative result and a low sensitivity. Since the smallest lymph node metastasis identified during surgery was 8 mm in size on imaging, the authors suggest a systematic approach by sampling of all mediastinal and hilar lymph nodes larger than 5 mm, in order to reduce upstaging after surgical procedure (14).
Billé and colleagues analysed 1,001 nodes in 159 patients before lung resection. Of 71 nodes that were found to be positive at histology, PET/CT correctly identified 41 metastatic lymph node stations (57.7%; 19 N1, 22 N2). False negative results were obtained in 30 nodal stations (5 N1, 24 N2, 1 N3), and false positive results in 14 (5 N1, 9 N2). The most common lymph node station for occult metastatic involvement was the subcarinal level (26.6%) followed by right upper and lower paratracheal, and hilar levels (4 each out of 30) (2,15).
The development of new techniques can be integrated and employed to increase the diagnostic power of EBUS-TBNA: in 2020, a multicentre study was published reporting the results of EBUS strain elastography to help predict malignant lymph node involvement. The authors designed different scenarios combining PET results, lymph node size at EBUS, and EBUS-strain elastography, and found that the latter always increased the ability to detect positive lymph nodes (16).
Preoperative staging of the mediastinum in cN1 disease is crucial in reason of the high prevalence of occult N2/N3 disease (reported to be 20–42%): sampling of nodes from N3 to N1 station is recommended to avoid false diagnosis of higher stage of lung cancer (17).
Moreover, current guidelines suggest once the mediastinal staging is completed, sampling of hilar and interlobar N1 nodes with EBUS should be performed as relevant for induction therapy and for non-surgical patients considered for stereotactic body radiotherapy.
In an article reporting a ten-year experience with EBUS-TBNA, Rosso et al. recorded a 96% diagnostic accuracy; moreover, in 56 cases a molecular analysis was performed, and 98.2% of those samples resulted adequate for molecular testing (18).
EBUS-TBNA does not provide access to stations 5, 6, 8 and 9, while the trans-oesophageal approach (EUS), allows reaching of para-oesophageal and pulmonary ligament stations (8 and 9). For this reason, the two procedures, combined, provide an almost complete echo-endostaging of the mediastinum (1). Hwangbo et al. evaluated the role of EUS-FNA for mediastinal lymph nodes that were inaccessible or difficult to access by EBUS-TBNA, with a reference standard of surgical confirmation. The sensitivity, NPV, and diagnostic accuracy of EBUS-TBNA metastasis were 84.4%, 93.3%, and 95.1%, respectively: these results increased to 91.1%, 96.1%, and 97.2%, respectively, for the combination of EBUS-TBNA plus EUS-FNA. EUS allowed to identify mediastinal metastasis in three additional patients (19). Even though data show EBUS-TBNA plus EUS-FNA have higher accuracy in detecting mediastinal metastasis, today the use of combined ultrasound techniques is reserved to patients with high suspicion of station 8 and 9 involvement based on radiological imaging.
According to international guidelines, video-assisted thoracic surgery (VATS) approach should be preferred to anterior mediastinotomy for sampling of paraaortic and pre-vascular stations (stations 5 and 6) since several studies showed that sensitivity and accuracy of VATS is almost 100% for diagnosis and staging, with low morbidity and mortality.
Our centre’s experience
In our centre, EBUS-TBNA is performed in case of suspected or proven lung cancer with mediastinal lymph node involvement; suspected or proven centrally located lung tumours with or without mediastinal lymph node involvement; metastatic disease, whenever the metastasis cannot be biopsied. A contrast-enhanced computerized tomography (CT scan) of the chest and PET (CT-PET) are obtained in all patients, except for those affected by chronic renal failure or uncontrolled diabetes. Head CT scan is also performed in all patients for complete oncological staging, prior to or following the endoscopic procedure. Pre-procedure routine tests include a complete blood count, clotting tests and an electrocardiogram. All EBUS-TBNA procedures in our centre are performed in a dedicated operating room, in local anaesthesia and deep sedation in spontaneous breathing, with the support of an anaesthesiologist. In our hospital, EBUS-TBNA is always performed by a thoracic surgeon with an extensive endoscopic experience, assisted by a thoracic surgery trainee, and a dedicated nurse. Patients with an ECOG performance status lower than 2, are generally discharged the same day of procedure. Our equipment includes an EB19J10U ultrasound Video Bronchoscopy (Pentax Medical), an ultrasound platform Arietta V70A (Hitachi Medical System), and a 22G nitinol-made disposable needle (Medi-Globe). During the procedure, mediastinal and hilar node stations are systematically assessed using endoscopic ultrasound. Selective sampling of lymph nodes is then performed, based on nodes dimensions on CT scan, PET positivity, morphology at ultrasound, and vascular pattern. Nodal sampling is carried out starting with level of N3 nodes, followed by N2 and then N1 station (or to T, in case of central lesions), in order to avoid false diagnosis of higher stage due to needle contamination. We do not employ rapid on-site evaluation (ROSE) during the procedure. Cytological samples are stored in 10% neutral buffered formalin (NBF) to obtain the paraffin-embedded clot at 60 °C (cellblock) used for immunohistochemical examination for detection and characterization of tumours. We retrospectively analysed data regarding EBUS-TBNA performed in our centre from April 2016 to March 2020. The presence of benign disease or lymph nodes localization of tumour different from lung cancer, were exclusion criteria. We collected data about patients features, staging disease, intraoperative variables, complications and cytological examination. Post-operative bleedings were classified as major and moderate respectively, when orotracheal intubation and/or blood transfusion were needed, when only suction and instillation of cold saline was required and minor in case of self-limiting bleeding. During the study period, 456 EBUS-TBNA were performed at our institution. At final histological examination (surgery resection, diagnosis on other disease location, transthoracic needle aspiration biopsy) 186 patients were diagnosed with non-neoplastic disease, while 270 patients had a final diagnosis of cancer, 24 of which were metastasis (Table 1).
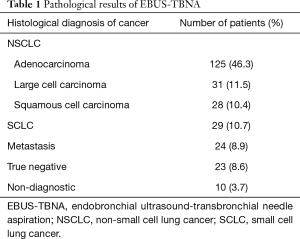
Full table
The median age of patients at the time of diagnosis was 66.8 years. The prevalence of male gender, smokers, and previous history of malignancy were 60.7% (164 male/106 female), 79.3% [214] and 13.3% [36], respectively.
Patient characteristics are shown in Table 2.
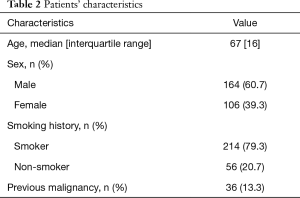
Full table
The clinical staging (cTNM) for NSCLC, according to 8th edition of TNM classification of lung cancer (20) at presentation was, in increasing order of incidence IB (n=3), IIA (n=1), IIB (n=1), IIIA (n=34), IIIB (n=46), IIIC (n=1), IVA (n=34), IVB (n=46). With regard to small cell lung cancer, 10 patients presented with an extended disease, 8 with a localized disease. We analysed 270 EBUS-TBNA performed for diagnostic and staging purpose through a retrospective study. The mean duration of procedure was 34 min (range, 20 to 65 min). In 28 patients we performed a diagnostic bronchoscopy for histological sampling at the same time. The mean number of sampled lymph node stations was 2.4 per procedure, and the mean of samples per station was 2.8. The mean quantity of extracted DNA for molecular testing was 1.34 µg (range, 300 ng–2,5 µg), while the mean number of sections obtained for immunocytochemistry was 6.3 (range, 4–8 sections). In our population the average diagnostic yield of EBUS-TBNA was 97.12% (236 out of 243 patients) for diagnosis of primary lung cancer and 96.30% (260 out of 270) for malignancy. In 10 patients, the cytological exam was non-diagnostic. In those cases, patients underwent minimally invasive surgery (VATS), EUS or fine-needle aspiration biopsy (FNAB) to obtain diagnosis. EBUS-TBNA sensitivity, specificity, PPV and NPV for detecting malignancy was 96%, 100%, 100% and 76% respectively. Out of 270 subjects with a definitive diagnosis of malignancy, 142 needed molecular testing [epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), c-ros oncogene 1 (ROS1)] or immunocytochemistry to determine programmed death ligand-1 (PD-L1) expression: the adequacy of the harvested samples was reached in 78.87% of cases (112 out of 142 patients). We found EGFR mutations in 19 patients (16.9%), ROS1 and ALK rearrangements in 1 and 2 patients respectively (0.9% and 1.8%). PD-L1 expression was higher than 1% in 39 patients (34.8%). In 5 patients we found more than one mutation. No significant complication occurred after EBUS-TBNA, except for one case of major complication in a male patient who developed tension pneumoperitoneum associated with severe haemodynamic instability. This rare complication required emergency intubation and decompression with a Veress needle, followed by admission to the intensive care unit for one night. Only one case of isolated tension pneumoperitoneum during EBUS-TBNA has been previously described (21). We hypothesized that the cause in this case could have been positive pressure ventilation. The most frequent complication in our study group was bleeding (3 cases): most of our patients had a minor intraoperative bleeding, and only one major bleeding which was treated with instillation of cold saline solution and adrenaline. Other complications reported were pneumothorax (1 case), desaturation (1 case), and low tolerance to procedure (2 cases). No patient required endotracheal ventilation nor transfusion of packed red blood cells during the procedure or in the postoperative period; there was no evidence of procedure-related deaths within 30 days after discharge.
Discussion
The heterogeneity in the diagnostic accuracy found in different reports is attributable to many factors. First of all, the definition of diagnosis varies among studies: samples obtained by EBUS-TBNA may be adequate to determine the presence of a tumour, but not enough to perform immunocytochemistry or molecular tests, which are often required to establish a correct target therapy. In fact, EBUS-TBNA has been progressively employed in patients with a locally advanced disease, to obtain a diagnosis and simultaneously establish the correct targeted therapy without requiring further invasive procedures. The accuracy is also affected by the operators’ expertise: EBUS-TBNA is usually performed by thoracic surgeons and interventional pulmonologists with experience in thoracic endoscopy. However, the presence of endoscopists still in their learning period, particularly in teaching hospitals, may influence the results of the studies. Moreover, operators with an extensive experience in EBUS-TBNA are able to describe the lymph nodes ultrasonographic features, including vascular and grayscale patterns, which is useful in case of equivocal cytology to support a diagnosis of benign tissue (2). EBUS-TBNA is also used in case of mediastinal restaging after induction therapy in patients with locally advanced disease. Some of the N2 patients undergone to induction chemotherapy o chemoradiation can successfully respond to treatment and be candidate surgical resection: for these cases, it is necessary to evaluate the real pathological response to treatment by mediastinal restaging. In a recently published meta-analysis, EBUS-TBNA was confirmed as an adequately accurate and safe technique for restaging, despite a decreased sensitivity compared to primary staging (22). Chemotherapy and radiotherapy can lead to lymph nodes necrosis and fibrosis, which make sampling and pathologic evaluation more demanding and increase the number of false-negative cases. On these grounds, as suggested by the last European Society of Gastrointestinal Endoscopy (ESGE), European Respiratory Society (ERS), ESTS guidelines, a negative sample obtained by endoscopic mediastinal restaging requires subsequent surgical restaging (1). When comparing the diagnostic accuracy of EBUS-TBNA with that of surgical procedures, it should also be considered that different lymph node sampling techniques are employed. During surgery, a systematic approach to lymph node stations is recommended, while in many centres EBUS-TBNA is performed using a “targeted approach”, where only nodes with abnormal radiological or ultrasonographic characteristics are biopsied. This could lead to the “missed lymph node” effect, with a higher frequency of false negative cases and a reduced sensitivity of EBUS-TBNA (14). On the other hand, a systematic approach during EBUS-TBNA would obviously result in a prolonged procedure time. Rapid on site cytologic evaluation (ROSE) improves the diagnostic accuracy of EBUS-TBNA, allowing to ensure adequate samples are obtained for pathological analyses (Izumo, Chen). ROSE is preferentially performed by a cytopathologist or cytotechnologist; however, since they are not always available during the endoscopic procedure, some centres prefer to train thoracic surgeons and interventional pulmonologists to perform ROSE instead. In a recently published retrospective analysis, Lin et al. reported their experience with ROSE: their results showed adequate on-site interpretation, which was carried out by trained pulmonologists, and an improvement in the diagnostic accuracy of EBUS-TBNA when combined with ROSE (23).
Conclusions
EBUS-TBNA is a safe procedure for hilar and mediastinal lymph nodes diagnosis and staging; it is less invasive than mediastinoscopy, and has excellent accuracy for the diagnosis of lung cancer. The results of our centre’s experience confirm these data, as we reported a 97.11% accuracy for the diagnosis of primary lung cancer and 96.29% for malignancy, with few severe complications. In our series, EBUS-TBNA yield adequate samples for molecular testing and immunocytochemistry in 78.87% of cases.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Angelo Carretta) for the series “Minimally Invasive (Endoscopic) Mediastinal Staging of Lung Cancer” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-21-2). The series “Minimally Invasive (Endoscopic) Mediastinal Staging of Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vilmann P, Frost Clementsen P, Colella S, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2015;48:1-15. [Crossref] [PubMed]
- Fielding DI, Kurimoto N. EBUS-TBNA/Staging of Lung Cancer. Clin Chest Med 2013;34:385-94. [Crossref] [PubMed]
- Jalil BA, Yasufuku K, Khan AM. Uses, Limitations, and Complications of Endobronchial Ultrasound. Proc (Bayl Univ Med Cent) 2015;28:325-30. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Herth F, Ernst A, Schulz M, et al. Endobronchial ultrasound reliably differentiates between airway infiltration and compression by tumor. Chest 2003;123:458-62. [Crossref] [PubMed]
- Erer OF, Erol S, Anar C, et al. Diagnostic yield of EBUS-TBNA for lymphoma and review of the literature. Endosc Ultrasound 2017;6:317-22. [Crossref] [PubMed]
- Wahidi MM, Herth F, Yasufuku K, et al. Technical aspects of endobronchial ultrasound-guided transbronchial needle aspiration CHEST guideline and expert panel report. Chest 2016;149:816-35. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-e250S.
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive Summary. Chest 2013;143:7S-37S. [Crossref] [PubMed]
- Recent Updates to NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Non-Small Cell Lung Cancer Version 8.2020. 2021;19462. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Postmus PE, Kerr KM, Oudkerk M, et al. Early-stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO clinical practice guidelines. Ann Oncol 2017;28:iv1-21. [Crossref] [PubMed]
- Ernst A, Anantham D, Eberhardt R, et al. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol 2008;3:577-82. [Crossref] [PubMed]
- Um SW, Kim HK, Jung SH, et al. Endobronchial ultrasound versus mediastinoscopy for mediastinal nodal staging of non-small-cell lung cancer. J Thorac Oncol 2015;10:331-7. [Crossref] [PubMed]
- Murthi M, Donna E, Arias S, et al. Diagnostic Accuracy of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (EBUS-TBNA) in Real Life. Front Med (Lausanne) 2020;7:118. [Crossref] [PubMed]
- Billé A, Pelosi E, Skanjeti A, et al. Preoperative intrathoracic lymph node staging in patients with non-small-cell lung cancer: accuracy of integrated positron emission tomography and computed tomography. Eur J Cardiothorac Surg 2009;36:440-5. [Crossref] [PubMed]
- Verhoeven RLJ, Trisolini R, Leoncini F, et al. Predictive Value of Endobronchial Ultrasound Strain Elastography in Mediastinal Lymph Node Staging: The E-Predict Multicenter Study Results. Respiration 2020;99:484-92. [Crossref] [PubMed]
- Leong TL, Loveland PM, Gorelik A, et al. Preoperative staging by ebus in cn0/n1 lung cancer: Systematic review and meta-analysis. J Bronchology Interv Pulmonol 2019;26:155-65. [Crossref] [PubMed]
- Rosso L, Ferrero S, Mendogni P, et al. Ten-year experience with endobronchial ultrasound-guided transbronchial needle aspiration: single center results in mediastinal diagnostic and staging. J Thorac Dis 2017;9:S363-9. [Crossref] [PubMed]
- Hwangbo B, Lee GK, Lee HS, et al. Transbronchial and transesophageal fine-needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer. Chest 2010;138:795-802. [Crossref] [PubMed]
- Detterbeck FC. The eighth edition TNM stage classification for lung cancer: What does it mean on main street? J Thorac Cardiovasc Surg 2018;155:356-9.
- Muriana P, Carretta A, Ciriaco P, et al. Isolated tension pneumoperitoneum following endobronchial ultrasound-guided transbronchial needle aspiration complicated by cardiac peri-arrest: A case report. Monaldi Arch Chest Dis 2018;88:999. [Crossref] [PubMed]
- Jiang L, Huang W, Liu J, et al. Endosonography with lymph node sampling for restaging the mediastinum in lung cancer: A systematic review and pooled data analysis. J Thorac Cardiovasc Surg 2020;159:1099-1108.e5. [Crossref] [PubMed]
- Lin CK, Jan IS, Yu KL, et al. Rapid on-site cytologic evaluation by pulmonologist improved diagnostic accuracy of endobronchial ultrasound-guided transbronchial biopsy. J Formos Med Assoc 2020;119:1684-92. [Crossref] [PubMed]
Cite this article as: Torre M, Reda M, Musso V, Danuzzo F, Mohamed S, Conforti S. Diagnostic accuracy of endobronchial ultrasound-transbronchial needle aspiration (EBUS-TBNA) for mediastinal lymph node staging of lung cancer. Mediastinum 2021;5:15.


