
Thoracic SMARCA4-deficient undifferentiated tumor—a case of an aggressive neoplasm—case report
Introduction
Thoracic SMARCA4-deficient undifferentiated tumors (SMARCA4-UT, formerly called “SMARCA4-deficient thoracic sarcoma”) are very aggressive tumors which most commonly occur in the mediastinum of male smokers (1-3). These tumors are characterized by an inactivating mutation in the SMARCA4 gene which encodes brahma-related gene 1 (BRG1). BRG1, a tumor suppressor, is part of the human switch/sucrose non-fermentable (SWI/SNF) complex [BRG1/BRM associated factor (BAF) complex] which is involved in chromatin remodeling (3-6).
SMARCA4-UT are characterized by a poorly differentiated morphology with at least a subset of these tumors exhibiting focal rhabdoid features. While the immunophenotype can vary, in general these tumors show no or only focal keratin expression (2,7,8). Loss of expression of BRG1 is the hallmark of these tumors. The pathogenesis is not entirely clear. Evidence suggests that these tumors might represent de-differentiated non-small cell carcinomas (2) although some features indicate that they are more closely related to other BAF-deficient tumors such as malignant rhabdoid tumor and small cell carcinoma of the ovary, hypercalcemic type (SCCOHT) (3). The diagnosis of these tumors is important as preclinical and early clinical trials using enhancer of zeste homolog (EZH2) inhibitors are promising (3,9).
Herein a case of a SMARCA4-UT is presented. This case illustrates typical demographic, morphologic and immunophenotypical features of these tumors. In addition, the tumor cells in this case focally express CD30. A review of the literature on SMARCA4-UT is also provided.
I present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/med-20-15).
Case presentation
A 66-year-old male is found to have a left suprahilar mass and additional masses in the mediastinum, right lung, liver, and right adrenal gland. His past medical history is remarkable for smoking and emphysema.
A needle core biopsy of an ‘anterior mediastinal lymph node’ shows extensive necrosis with scattered clusters and small sheets of medium-sized atypical epithelioid cells (Figure 1A). Lymphoid tissue is not identified. The atypical cells are characterized by a moderate amount of cytoplasm (Figure 1B). The nuclei are round to oval, have open chromatin and prominent nucleoli. Mild cellular pleomorphism is identified. Some neoplastic cells have rhabdoid features (Figure 1C). The neoplastic cells are somewhat loosely arranged with focal more pronounced discohesive growth pattern. Gland formation, cytoplasmic vacuolization, or keratinization are not identified. Based on morphologic features and location of the mass the differential diagnosis includes non-small cell carcinoma, sarcoma, malignant melanoma, lymphoma, and malignant mesothelioma. Immunostains show that the tumor cells lack expression of multiple keratins including Oscar keratin (Figure 1D), pankeratin, high molecular weight keratin, CK5/6, CK7, and CK20, essentially excluding carcinoma and malignant mesothelioma. A lymphoma workup (including CD3, CD20, CD45) is also negative. The tumor cells are focally positive for CD30 (Figure 1E), and focally weakly for CD138 while they lack staining with p63, TTF 1, napsin, CD31, S100, calretinin, D2-40, WT-1, NUT, SALL-4, myogenin, desmin, and SMA.
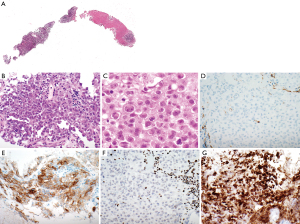
Given the clinical presentation of an aggressive, widely metastatic tumor, together with the focal rhabdoid morphology and the immunophenotype, a SMARCA4-UT is considered. Therefore, a BRG1 immunostain is performed which shows loss of expression of BRG1 with an intact positive internal control (Figure 1F). INI-1 expression is preserved (Figure 1G). This immunoprofile supports a diagnosis of SMARCA4-UT. Follow-up is not available in this patient.
The informed consent was waived as this was a case of the consultation files of the author. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Discussion
SMARCA4-UT are aggressive neoplasms. The patient presented has widespread metastatic disease at time of diagnosis. Similarly, literature reports metastases at time of presentation in the majority of patients including to lymph nodes (59% to 91% of patients), adrenal gland (27% to 48%), lung (29%), and bone (24% to 55%) (1,2). In the presented patient, metastatic disease is also identified in the liver. The demographic features of the described patient are consistent with reports of other patients with SMARCA4-UT with median age between 39 and 59 (range, 27 to 82) years old (1,2,5,7,8). There is a male predominance with a male to female ratio of up to 9:1. Most patients are smokers. Patients most commonly present with dyspnea, chest pain, and superior vena cava syndrome (1). Tumors are often large at presentation with a reported median tumor size of 12 to 13 (range, 2.2 to 27) cm. Tumors are most commonly found in the anterior mediastinum followed by pleura and lung.
CT imaging studies of SMARCA4-UT describe four main tumor patterns with the mediastinal pattern being the most common (61%) followed by pleural (29%), cervical (5%), and retroperitoneal (5%) patterns (1). Imaging features of these tumors include ill-defined margins and heterogeneous enhancement after contrast. Multicompartment extension from the mediastinum to lung apex, pleura, or neck, compression of large airways resulting in post-obstructive lung atelectasis, vascular encasement and compression resulting in superior vena cava syndrome, and esophageal invasion is reported in a subset of cases. Primary tumors exhibit strong FDG avidity on PET-CT scan (1,2).
Morphologically, as seen in the presented case, these tumors are characterized by poorly differentiated cytology with medium-sized epithelioid cells with irregular nuclear borders and often conspicuous nucleoli. Sometimes, at least focally, a rhabdoid morphology can be appreciated as also seen in this case. Focal myxoid stroma or desmoplastic small round cell tumor-features are described in 7% of the tumors (8). Mitotic activity is high. While the tumor cells are forming clusters and sheets, they are growing in a somewhat discohesive pattern. Usually, extensive necrosis is also present.
The immunoprofile of these tumors can vary. In the presented case the tumor cells are focally positive for CD30 and CD138 while being negative for many other markers including various keratins. Many of these tumors are negative for multiple keratins as in our case; only in some of these cases tumor cells are reported to be focally positive for keratin and EMA. Diffuse expression of Sox2 is reported in almost all cases (3,5,8). About one-third of the cases are reported to express CD34 and/or SALL4. Synaptophysin might also be expressed in a subset of cases. Rare tumors focally express claudin 4 and TTF-1. As in the case presented, SMARCA4-UT are negative for desmin, NUT, S100, WT-1, and p40 (2,7,8). In 69% to 88% of cases a TP53 mutation is identified (2,3).
SMARCA4 encodes BRG1, a tumor suppressor, which is a catalytic subunit of the SWI/SNF (BAF) chromatin-remodeling complex. The SWI/SNF complex is involved in the regulation of transcription and promotion of cell differentiation (3-5). SMARCA4-UT are characterized by inactivating mutations of SMARCA4 resulting in loss of BRG1 expression in neoplastic cells (6). SMARCA4 germline mutations are not identified in these patients. In general, these tumors also show loss of BRM-1 (encoded by SMARCA2, another catalytic subunit of SWI/SNF) although a study of SMARCA4-UT in the lung showed preserved expression in 18% of the cases (2,7).
The pathogenesis of SMARCA4-UT is not entirely clear. It is still debated whether these tumors represent de-differentiated lung adenocarcinomas or might be more related to BAF-deficient rhabdoid tumors such as malignant rhabdoid tumors or atypical teratoid/rhabdoid tumors and SCCOHT. The latter is supported for instance by an RNA sequencing study that clustered cases into (I) carcinomas (renal medullary carcinoma with loss of SMARCB1 and lung carcinomas with or without SMARCA4 mutation), (II) unclassified and epithelioid sarcoma, and (III) SMARCA4-UT, SCCOHT, and malignant rhabdoid tumors (3). Furthermore, in that study, more than 450 genes are differentially expressed between SMARCA4-UT and lung carcinoma with SMARCA4 mutation and unclassified thoracic sarcomas with wild type SMARCA4. In addition, low/deficient mRNA expression of SMARCA2 is observed in SMARCA4-UT, SCCOHT, and SMARCA4-deficient malignant rhabdoid tumors in contrast to preserved expression in lung carcinomas and sarcomas. Moreover, morphological features are similar between SMARCA4-UT, SCCOHT, and malignant rhabdoid tumor. More recent studies show evidence in support of SMACRA4-UT possibly representing de-differentiated lung carcinomas. Indeed, no germ line mutations are identified in SMARCA4-UT in contrast to 35% of SMARCB1- or SMARCA4-deficient malignant rhabdoid tumors and 50% of SMARCA4-deficient SCCOHT that harbor germ line mutations (3). Furthermore, similar to lung carcinomas, SMARCA4-UT have a complex genetic profile. In addition, these tumors can primarily affect the lung, a subset of these tumors has a component of a conventional non-small cell carcinoma, they frequently occur in smokers, and 50% of the cases harbor alterations typical of smoking-related non-small cell carcinomas including mutations in STK11, KEAP1, KRAS, and NF1 (2). Also, BAF complex alterations are present in a subset of undifferentiated/de-differentiated carcinoma. Indeed, 4% to 6% of lung adenocarcinomas are SMARCA4 deficient (10,11).
The differential diagnosis of SMARCA4-UT includes malignant rhabdoid tumor and atypical teratoid/rhabdoid tumor, metastatic SCCOHT, lung carcinoma, NUT carcinoma, germ-cell tumor, lymphoma, malignant melanoma, malignant mesothelioma, and sarcoma (8). Loss of BRG1 expression by itself is not specific for SMARCA4-UT as it can also be seen in carcinomas including of lung origin, SCCOHT, and a subset of malignant rhabdoid tumors and atypical teratoid/rhabdoid tumors. Moreover, malignant rhabdoid tumors, atypical teratoid/rhabdoid tumors, and SCCOHT share similar morphological features with SMARCA4-UT. However, patients with malignant rhabdoid tumor are usually younger and tumors are negative for Sox2, do not show p53 overexpression, and commonly lack expression of INI-1 (8). Patients with SCCOHT are females and have a history of a primary intra-abdominal tumor with or without hypercalcemia (8). Although Sox2 might be expressed in these tumors, expression is usually not diffuse. In addition, INI-1 (encoded by SMARCB1 gene, another member of the SWI/SNF complex) immunostain can be helpful to further exclude tumors that have similar morphologic features but characteristically show loss of INI-1 expression such as epithelioid sarcoma (in which BRG1 expression is preserved).
The median survival of patients with SMARCA4-UT is reportedly very short with only 4 to 7 (range, 1 to 13) months. Disease progression or relapse occurs in essentially all patients and patients in general die of their disease, mostly due to local complications (1,3). Response to chemotherapy and surgery is limited (1). However, promising preclinical studies and early clinical trials with the H3K27 histone methyltransferase EZH2 inhibitor are underway in tumors related to the SWI/SNF complex (NCT03213665; NCT02875548; NCT02601950) emphasizing the importance of an accurate diagnosis of this tumor (3,9). In addition, a few patients with SMARCA4-UT are reported who were treated with pembrolizumab, an anti-PD-1 antibody, either as first-line therapy or after failed chemotherapy and showed prolonged partial response (12,13). One of these tumors expressed PD-L1 on 60% of the tumor cells (13), in the other case PD-L1 expression was not reported (12).
In conclusion, SMARCA4-UT are very aggressive tumors that occur predominantly in male smokers. While the pathogenesis is still investigated evidence suggests that these tumors might represent de-differentiated lung adenocarcinomas. Early preclinical and clinical trials using EZH2 inhibitors are underway. This diagnosis should be considered in the differential diagnosis of poorly differentiated epithelioid thoracic tumors.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mirella Marino, Brett W. Carter) for the series “Dedicated to the 10th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2019)” published in Mediastinum. The article has undergone external peer review.
Reporting Checklist: The author has completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/med-20-15
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-15). The series “Dedicated to the 10th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2019)” was commissioned by the editorial office without any funding or sponsorship. ACR serves as an unpaid Associate Editor of Mediastinum from May 2017 to June 2021.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The informed consent was waived as this was a case of the consultation files of the author. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Crombé A, Alberti N, Villard N, et al. Imaging features of SMARCA4-deficient thoracic sarcomas: a multi-centric study of 21 patients. Eur Radiol 2019;29:4730-41. [Crossref] [PubMed]
- Rekhtman N, Montecalvo J, Chang JC, et al. SMARCA4-deficient thoracic sarcomatoid tumors represent primarily smoking-related undifferentiated carcinomas rather than primary thoracic sarcomas. J Thorac Oncol 2020;15:231-47. [Crossref] [PubMed]
- Le Loarer F, Watson S, Pierron G, et al. SMARCA4 inactivation defines a group of undifferentiated thoracic malignancies transcriptionally related to BAF-deficient sarcomas. Nat Genet 2015;47:1200-5. [Crossref] [PubMed]
- Schick S, Rendeiro AF, Runggatscher K, et al. Systematic characterization of BAF mutations provides insights into intracomplex synthetic lethalities in human cancers. Nat Genet 2019;51:1399-410. [Crossref] [PubMed]
- Yoshida A, Kobayashi E, Kubo T, et al. Clinicopathological and molecular characterization of SMARCA4-deficient thoracic sarcomas with comparison to potentially related entities. Mod Pathol 2017;30:797-809. [Crossref] [PubMed]
- Schaefer IM, Cote GM, Hornick JL. Contemporary sarcoma diagnosis, genetics, and genomics. J Clin Oncol 2018;36:101-10. [Crossref] [PubMed]
- Sauter JL, Graham RP, Larsen BT, et al. SMARCA4-deficient thoracic sarcoma: a distinctive clinicopathological entity with undifferentiated rhabdoid morphology and aggressive behavior. Mod Pathol 2017;30:1422-32. [Crossref] [PubMed]
- Perret R, Chalabreysse L, Watson S, et al. SMARCA4-deficient thoracic sarcomas: clinicopathologic study of 30 cases with an emphasis on their nosology and differential diagnoses. Am J Surg Pathol 2019;43:455-65. [Crossref] [PubMed]
- Chan-Penebre E, Armstrong K, Drew A, et al. Selective killing of SMARCA2- and SMARCA4-deficient small cell carcinoma of the ovary, hypercalcemic type cells by inhibition of EZH2: in vitro and in vivo preclinical models. Mol Cancer Ther 2017;16:850-60. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543-50. Erratum in: Nature 2014;514:262 Erratum in: Nature 2018;559:E12. [Crossref] [PubMed]
- Nambirajan A, Singh V, Bhardwaj N, et al. SMARCA4/BRG1-deficient non-small cell lung carcinomas: a case series and review of the literature. Arch Pathol Lab Med 2021;145:90-8. [Crossref] [PubMed]
- Henon C, Blay JY, Massard C, et al. Long lasting major response to pembrolizumab in a thoracic malignant rhabdoid-like SMARCA4-deficient tumor. Ann Oncol 2019;30:1401-3. [Crossref] [PubMed]
- Takada K, Sugita S, Murase K, et al. Exceptionally rapid response to pembrolizumab in a SMARCA4-deficient thoracic sarcoma overexpressing PD-L1: A case report. Thorac Cancer 2019;10:2312-5. Erratum in: Thorac Cancer 2020;11:3645. [Crossref] [PubMed]
Cite this article as: Roden AC. Thoracic SMARCA4-deficient undifferentiated tumor—a case of an aggressive neoplasm—case report. Mediastinum 2021;5:39.


