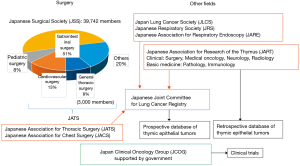
System for thymic disease research and clinical practice in Japan
Several academic associations in Japan have been working together to research thymic diseases and apply those results to clinical practice, with the relationships of these associations and their functions shown in Figure 1.
The Japanese Surgical Society (JSS) is the largest association in the field of surgery in Japan and covers all fields, including gastrointestinal, liver, cardiac, general thoracic, breast, endocrine organ surgery, and pediatric surgery, as well as traumatology, with 39,742 members in 2019. General thoracic surgery accounts for 8% of the JSS, while they also belong to both the Japanese Association for Chest Surgery (JACS) and Japanese Association for Thoracic Surgery (JATS), with the latter group covering all fields of thoracic surgery (cardiovascular, esophageal, lung, mediastinal). Thus, active surgeons treating thymic diseases are members of JSS, JACS, and JATS concurrently.
The Japan Lung Cancer Society (JLCS) was formed for physicians in all fields who treat lung cancer and thoracic malignancies. Also, the Japanese Association for Research of the Thymus (JART) is a cross-sectional association of physicians and researchers in the fields of thoracic surgery, medical oncology, neurology, radiology, pathology, and basic immunology, with 550 members in 2018. Most general thoracic surgeons and medical oncologists involved in treating lung cancer cases are also members of the Japanese Respiratory Society (JRS) and Japanese Association for Respiratory Endoscopy (JARE).
JACS, JATS, JLCS, JRS, JARE, JART, and the Japan Asbestos Mesothelioma Interest Group (JAMIG) jointly operate the Japanese Joint Committee for Lung Cancer Registry (JJCLCR), which has constructed and maintains a nationwide database of lung cancer, thymic epithelial tumor, and malignant mesothelioma cases. The Japan Clinical Oncology Group (JCOG), which is independent of these academic associations, is supported by the Japanese government and conducts clinical trials, with JCOG 9605 and JCOG 9606 reported by Kunitoh et al. (1,2).
Based on the advocacy of Prof. Akira Masaoka at Nagoya City University, JART was founded in 1982. Annual meetings are conducted, with the 39th meeting hosted by Prof. Atsushi Watanabe in Sapporo, Hokkaido, in 2019, during which 60 abstracts were presented. The “General Rules for Study of Mediastinal Tumors” was edited by the former president of JART, Professor Emeritus Yoshitaka Fujii at Nagoya City University, in 2009 (Figure 2).
JART has initiated three clinical trials. JART01, a prospective study of carboplatin + paclitaxel as chemotherapy for thymoma, was unfortunately discontinued because of the appearance of fatal myocarditis as a severe side effect (3). JART02 was conducted as a surgical trial to compare a partial with a subtotal thymectomy (4), while JART03, performed in collaboration with Lung Oncology Group in Kyushu (LOGIC), was a prospective study of cisplatin + TS1 + RT as chemoradiotherapy for locally advanced thymic carcinoma (5).
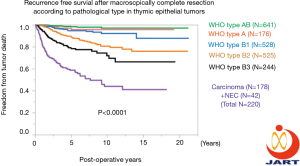
Another important achievement by JART was development of a nationwide retrospective database of 2,793 thymic epithelial tumor cases surgically treated in Japan between 1991 and 2010. Thirty-two experienced institutes participated in the project. Findings of recurrence-free survival after a macroscopically complete resection according to pathological type are shown in Figure 3. Ten research papers that used this database have been published in English language journals (6-15). The JART retrospective database also contributed to establishing UICC TNM staging in collaboration with ITMIG and IASLC (16). Presently, JART and JJCLCR are concurrently building a prospective database of thymic epithelial tumor cases.
JART has played an important role for communication with global societies. Further collaborations among Japanese societies and international associations are expected, and will contribute to advancement in research of thymic diseases.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mirella Marino, Brett W. Carter) for the series “Dedicated to the 10th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2019)” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-2020-04). The series “Dedicated to the 10th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2019)” was commissioned by the editorial office without any funding or sponsorship. MO serves as an unpaid editorial board member of Mediastinum from Jul 2019 to Jun 2021. The author has no other conflicts of interest to declare.
Ethical Statement: The authors is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kunitoh H, Tamura T, Shibata T, et al. A phase-II trial of dose-dense chemotherapy in patients with disseminated thymoma: report of a Japan Clinical Oncology Group trial (JCOG 9605). Br J Cancer 2009;101:1549-54. [Crossref] [PubMed]
- Kunitoh H, Tamura T, Shibata T, et al. A phase II trial of dose-dense chemotherapy, followed by surgical resection and/or thoracic radiotherapy, in locally advanced thymoma: report of a Japan Clinical Oncology Group trial (JCOG 9606). Br J Cancer 2010;103:6-11. [Crossref] [PubMed]
- Sasaki H, Yano M, Kawano O, et al. Thymoma associated with fatal myocarditis and polymyositis in a 58-year-old man following treatment with carboplatin and paclitaxel: A case report. Oncol Lett 2012;3:300-2. [Crossref] [PubMed]
- Yano M, Fujii Y, Yoshida J, et al. A Phase II Study of Partial and Subtotal Thymectomy for Thymoma (JART02). World J Surg 2017;41:2033-8. [Crossref] [PubMed]
- Fukuda M, Funaki S, Yamazaki T, et al. S-1 plus cisplatin with concurrent radiotherapy for locally advanced thymic carcinoma: Study protocol of LOGIK1605/JART-1501. Thorac Cancer 2020;11:693-6. [Crossref] [PubMed]
- Nakagawa K, Yokoi K, Nakajima J, et al. Is thymomectomy alone appropriate for stage I (T1N0M0) thymoma? Results of a propensity-score analysis. Ann Thorac Surg 2016;101:520-6. [Crossref] [PubMed]
- Agatsuma H, Yoshida K, Yoshino I, et al. Video-assisted thoracic surgery thymectomy versus sternotomy thymectomy in patients with thymoma. Ann Thorac Surg 2017;104:1047-53. [Crossref] [PubMed]
- Yamada Y, Yoshino I, Nakajima J, et al. Japanese Association for Research of the Thymus. Surgical outcomes of patients with stage III thymoma in the Japanese nationwide database. Ann Thorac Surg 2015;100:961-7. [Crossref] [PubMed]
- Okuda K, Yano M, Yoshino I, et al. Thymoma patients with pleural dissemination: nationwide retrospective study of 136 cases in Japan. Ann Thorac Surg 2014;97:1743-8. [Crossref] [PubMed]
- Omasa M, Date H, Sozu T, et al. Postoperative radiotherapy is effective for thymic carcinoma but not for thymoma in stage II and III thymic epithelial tumors: the Japanese Association for Research on the Thymus Database Study. Cancer 2015;121:1008-16. [Crossref] [PubMed]
- Hishida T, Nomura S, Yano M, et al. Long-term outcome and prognostic factors of surgically treated thymic carcinoma: Results of 306 cases from a Japanese Nationwide Database Study. Eur J Cardiothorac Surg 2016;49:835-41. [Crossref] [PubMed]
- Mizuno T, Okumura M, Asamura H, et al. Japanese Association for Research on Thymus. Surgical management of recurrent thymic epithelial tumors: a retrospective analysis based on the Japanese nationwide database. J Thorac Oncol 2015;10:199-205. [Crossref] [PubMed]
- Nakajima J, Okumura M, Yano M, et al. Japanese Association for Research of Thymus. Myasthenia gravis with thymic epithelial tumour: a retrospective analysis of a Japanese database. Eur J Cardiothorac Surg 2016;49:1510-5. [Crossref] [PubMed]
- Okumura M, Yoshino I, Yano M, et al. Tumour size determines both recurrence-free survival and disease-specific survival after surgical treatment for thymoma. Eur J Cardiothorac Surg 2019;56:174-81. [Crossref] [PubMed]
- Hamaji M, Sozu T, Machida R, et al. Mortality from extrathymic malignancy after thymic tumour resections: incidences and risk factors. Interact Cardiovasc Thorac Surg 2019;29:729-36. [Crossref] [PubMed]
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG thymic epithelial tumors staging project: Proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S65-S72. [Crossref] [PubMed]
Cite this article as: Okumura M. System for thymic disease research and clinical practice in Japan. Mediastinum 2021;5:7.