
Autogenous pericardial angioplasty for thymic malignancies: a narrative review
Introduction
Thymic epithelial tumor (TET) is a rare tumor mostly located in the anterior mediastinum with comparatively good prognosis (1). However, thymoma still has the ability to invade neighboring organs, such as superior vena cava (SVC) and innominate veins. Thymic tumors involving the adjacent great vessels are III-IV stage thymic malignancies, and the prognosis for those diseases is poor after conservative treatment (2). Therefore, locally advanced thymic tumors infiltrating mediastinum great vessels, when radically resectable, can be surgically performed by vessels prosthetic replacement within a multimodality therapeutic approach (3). Many materials have been widely reported for the reconstruction of mediastinal vessels, while the optimal treatment strategy for vessels resection and prosthetic replacement is still in controversial (4-6).
Nowadays, the polytetrafluoroethylene (PTFE) and bovine pericardial conduit are mainly used artificial vessels for SVC prosthetic replacement (7,8). Although the application of artificial conduit has expanded the surgical indications for invasive thymic tumors, the biological characteristics of immune rejection must be noticed after vascular conduit reconstruction.
In this study, we tried to use autologous pericardial graft for SVC and left innominate vein-right atrium by-pass reconstruction in the surgery of locally advanced thymic tumours, in order to reduce the graft-related complications compared with heterologous conduits. We here report the results of our experience in thymic malignancies en bloc radical resection with conduit reconstruction, and to analyze the short-term outcomes of this extended surgery.
We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/med-20-57).
Population
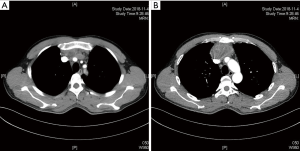
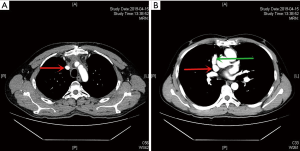
The diagnosis of thymic malignancies with mediastinal great vessels involvement mainly depends on imaging inspections such as computed tomography (CT) and magnetic resonance imaging (MRI). CT scan (especially chest dynamic enhanced CT) and MRI can clearly show the location and type of malignant thymoma invasion of the SVC and other neighboring organs (Figure 1), which is an important indication for tumor radically resectable and surgical procedures. Fludeoxyglucose positron emission tomography (FDG-PET) was performed before surgery for patients with suspected metastatic lesions found at CT-scan (9). The clinical and pathological characteristics of our case series were shown in Table 1.
Full table
Operative techniques
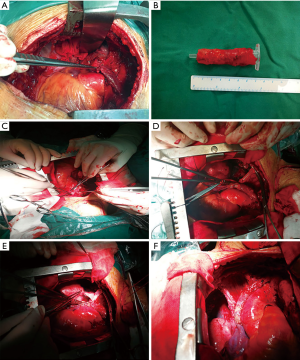
All the surgeries were performed through a median sternotomy. Indication for circumferential caval resection and conduit reconstruction was established when the tumour infiltrated more than 30% of the vessel circumference (10). After general anesthesia, the patients were placed in the supine position. Afterwards, the sternum was spread to explore the tumor location, infiltrating situation of the SVC and other adjacent organs. Then, the tumor-infiltrated pericardium was resected and the normal pericardial patch was prepared as large as possible. Meanwhile, the pericardial segment of SVC, left and right innominate veins were fully dissected (Figure 2A). Measuring the distance between the left innominate vein without tumor involvement and the auricula dextra cordis, then using autologous pericardium to make an artificial great vessel conduit (Figure 2B). The pericardium is opened as close to the hilum as possible in an attempt to avoid prosthetic patch repair of a large pericardial defect, but the opening must be generous enough to provide adequate exposure of the atrium and proximal cava. The vascular forcep was used to clamp the distal end of the left innominate vein, and the curved auricle clamp was also used to clamp part of the auricula dextra cordis. The left innominate vein-right atrium by-pass was built by autologous pericardial conduit, which sutured with 5-0 Polypropylene (Figure 2C). The thymic tumor and infiltrated vessels were radically resectred after blocking the distal right innominate vein and pericardial segment of SVC (Figure 2D). The autologous pericardium was used to reconstruct the SVC lateral wall or replacement SVC (basing on the degree of SVC tumor involvement) by 5-0 Polypropylene (Figure 2E). Before the final two stitches, opening the right innominate vein forceps and expelling the air in reconstructed vessels. Afterwards, the proximal vascular graft anastomosis is completed, making sure there is no anastomotic stenosis or anastomotic fistula (Figure 2F).
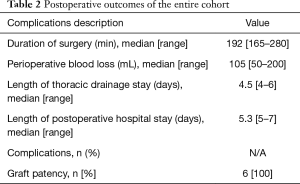
In this case series study, all the vessels involved were reconstructed with autologous pericardial grafts. Furthermore, after the autogenous replacement, patients would receive low-weight heparin for 3 days to reduce the risk of thrombosis, without receiving life-long anticoagulation therapy. All the patients had no complications such as long-term postoperative catheterization (>7 days), anastomotic bleeding, revascularization stenosis, embolism, and even unplanned secondary sternotomy (Table 2). The patency status of the reconstructed vessels was assessed by CT with volume rendering and contrast medium concurrently with the planned oncologic check (Figure 3).
Full table
Comments
Thymoma is the most common tumor of anterior mediastinum, accounting for 20–25% of all mediastinal tumors and 50% of the anterior mediastinal tumors, of which 20–30% are thymic malignancies with infiltrating the surrounding thoracic structures (11,12). Radical resection of the thymic tumors and infiltrated adjacent organs is an independent risk factor for improving the survival outcomes. However, the mediastinal great vessels must be reconstructed after achieved R0 resection during the operation (13,14).
Since the initial experiences, various autologous or heterologous materials for vascular reconstruction have been introduced and proposed. PTFE conduit is the widely used synthetic vascular prosthesis material for mediastinal great vessels replacement (6). However, due to the immune rejection of heterologous prosthesis, patients need a life-long anticoagulant treatment to prevent graft-related stenosis and thrombosis.While there are still some patients with recurrent SVC syndrome due to graft embolism. At the same time, the intravenous sodium heparin administrated before clamping and long-term administrated anticoagulant drugs after PTFE reconstruction has greatly increased the possibility of hemorrhage in the wound surface even stomas. Sun and his colleagues demonstrated that 32% of patients undergoing PTFE prosthesis replacement for thymic tumors required blood transfusion, and 4% of patients received secondary sternotomy for hemostasis due to conduit anastomotic hemorrhage (14). Other conduits including tubularized bovine pericardium, saphenous vein and more recently, porcine pericardium are also selectable graft (15). According to previous studies, the incidence of postoperative graft-related complications for thymic tumor is as high as 50%, which increases the economic and psychological burden of patients to a certain extent (3,16,17).
Based on the limited published data, the autologous pericardium has a lower risk of infection and thrombosis in comparison with synthetic materials, and does not need long-term anticoagulation, making it an ideal graft for vascular reconstruction (18-20). However, other previous studies have also shown that the reconstructed conduit is prone to restenosis after surgery, due to lack of effective lumen. Therefore, the best strategy for SVC replacement still remains controversial (6,10). In order to further improve the prognosis of patients with thymic malignancies and reduce the incidence of vascular graft-related complications, our center tried to use autologous pericardium graft for vessel reconstruction. The advantages of histocompatibility avoid the life-long use of anticoagulant drugs such as heparin and warfarin after surgery, thereby reducing the risk of graft-related anastomotic hemorrhage and thrombosis. In this case series, patients underwent related surgery have recovered well during the perioperative period, without graft-related complications. The planned postoperative CT-scan indicated no stenosis and intraluminal thrombosis in the reconstructed vessels. In our center, through reasonable planning of the surgical procedure before and during the operation, the autologous pericardium can meet the reconstruction requirements of the mediastinal vessels in most of the thymic malignancies. At present, we has routinely applied autologous pericardium to reconstruct mediastinal great vessels in patients with locally advanced thymic tumors for radically resection and improved survival outcomes.
In conclusion, en bloc radical resection and conduit reconstruction of mediastinal great vessels with autologous pericardium is a safe and effective procedure. Compared with heterologous grafts, autologous pericardial conduit reduces the high-risk complications related to vascular replacement. This technique is a favourite option for thymic malignancies in our experience.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/med-20-57
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-57). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siesling S, van der Zwan JM, Izarzugaza I, et al. Rare thoracic cancers, including peritoneum mesothelioma. Eur J Cancer 2012;48:949-60. [Crossref] [PubMed]
- Cardillo G, Carleo F, Giunti R, et al. Predictors of survival in patients with locally advanced thymoma and thymic carcinoma (Masaoka stages III and IVa). Eur J Cardiothorac Surg 2010;37:819-23. [Crossref] [PubMed]
- Berghmans T, Durieux V, Holbrechts S, et al. Systemic treatments for thymoma and thymic carcinoma: A systematic review. Lung Cancer 2018;126:25-31. [Crossref] [PubMed]
- Spaggiari L, Magdeleinat P, Kondo H, et al. Results of superior vena cava resection for lung cancer, Analysis of prognostic factors. Lung Cancer 2004;44:339-46. [Crossref] [PubMed]
- Chen KN, Xu SF, Gu ZD, et al. Surgical treatment of complex malignant anterior mediastinal tumors invading the superior vena cava. World J Surg 2006;30:162-70. [Crossref] [PubMed]
- Warren WH, Piccione WJ Jr, Faber LP. As originally published in 1990: Superior vena caval reconstruction using autologous pericardium. Updated in 1998. Ann Thorac Surg 1998;66:291-2. [Crossref] [PubMed]
- Van Putten JW, Schlosser NJ, Vujaskovic Z, et al. Superior vena cava obstruction caused by radiation induced venous fibrosis. Thorax 2000;55:245-6. [Crossref] [PubMed]
- Spaggiari L, Thomas P, Magdeleinat P, et al. Superior vena cava resection with prosthetic replacement for non-small cell lung cancer: long-term results of a multicentric study. Eur J Cardiothorac Surg 2002;21:1080-6. [Crossref] [PubMed]
- Spaggiari L, Casiraghi M, Guarize J. Multidisciplinary treatment of malignant thymoma. Curr Opin Oncol 2012;24:117-22. [Crossref] [PubMed]
- Maurizi G, Poggi C, D'Andrilli A, et al. Superior vena cava replacement for thymic malignancies. Ann Thorac Surg 2019;107:386-92. [Crossref] [PubMed]
- Cohen DJ, Ronnigen LD, Graeber GM, et al. Management of patients with malignant thymoma. J Thorac Cardiovasc Surg 1984;87:301-7. [Crossref] [PubMed]
- Large SR, Shneerson JM, Stovin PG, et al. Surgical pathology of the thymus: 20 years’ experience. Thorax 1986;41:51-4. [Crossref] [PubMed]
- Yagi K, Hirata T, Fukuse T, et al. Surgical treatment for invasive thymoma, especially when the superior vena cava is invaded. Ann Thorac Surg 1996;61:521-4. [Crossref] [PubMed]
- Sun Y, Gu C, Shi J, et al. Reconstruction of mediastinal vessels for invasive thymoma: a retrospective analysis of 25 cases. J Thorac Dis 2017;9:725-33. [Crossref] [PubMed]
- D'Andrilli A, De Cecco CN, Maurizi G, et al. Reconstruction of the superior vena cava by biologic conduit: assessment of long-term patency by magnetic resonance imaging. Ann Thorac Surg 2013;96:1039-45. [Crossref] [PubMed]
- Windisch T, Fischer JR, Vega A, et al. Infiltration of the superior vena cava in NSCLC: results of surgical intervention. Pneumologie 2015;69:23-9. [PubMed]
- Dai W, Dong J, Zhang H, et al. Superior vena cava replacement combined with venovenous shunt for lung cancer and thymoma: a case series. J Thorac Dis 2018;10:363-70. [Crossref] [PubMed]
- D'Andrilli A, Ibrahim M, Venuta F, et al. Glutaraldehyde preserved autologous pericardium for patch reconstruction of the pulmonary artery and superior vena cava. Ann Thorac Surg 2005;80:357-8. [Crossref] [PubMed]
- D’Andrilli A, Venuta F, Rendina EA. Surgical approaches for invasive tumors of the anterior mediastinum. Thorac Surg Clin 2010;20:265-84. [Crossref] [PubMed]
- Spaggiari L, Leo F, Veronesi G, et al. Superior vena cava resection for lung and mediastinal malignancies: a single-center experience with 70 cases. Ann Thorac Surg 2007;83:223-9; discussion 229-30. [Crossref] [PubMed]
Cite this article as: Gao HJ, Shi GD, Pan MJ, Liu XT, Wei YC. Autogenous pericardial angioplasty for thymic malignancies: a narrative review. Mediastinum 2021;5:6.


