
Sarcomas of the mediastinum with epithelioid morphology
Introduction
Although soft tissue sarcomas are typically associated with spindled, pleomorphic or round cells, certain types of sarcomas can have features amazingly similar to epithelial neoplasms. The tumor cells in these “epithelial-like” sarcomas can have rounded to ovoid nuclei which can be vesicular in nature. The cells can be arranged in a tubular or “glandular-like” arrangements frequently seen in carcinomas, mesotheliomas or thymic tumors. Moreover, these sarcomas frequently exhibit immunohistochemical staining for broad spectrum cytokeratins or epithelial membrane antigen (EMA), further emulating carcinomas. These overlapping features present a formidable diagnostic challenge to the pathologist who encounters a sarcoma with epithelioid morphology. When these tumors arise in locations removed from any epithelial structures (such as soft tissue of extremities for example), one can better consider a mesenchymal tumor with epithelioid morphology. As an anatomic site, however, the mediastinum hosts a wide variety of carcinomas which reflect the diversity of its organs and structures. Additionally, mesenchymal neoplasms comprise only a small percentage (approximately 5%) of primary tumors found in this location (1,2). In the mediastinum, a sarcoma with epithelioid features is much more likely to be mistaken for a carcinoma or mesothelioma. Consequently, a familiarity with the specific characteristics of sarcomas with epithelioid features is essential for a correct diagnosis.
When suspecting a diagnosis of sarcoma with epithelioid features, all attributes should be taken into consideration during evaluation. The clinical and radiologic attributes, such as the size and progression of the lesion, association with adjacent anatomic structures, and patient’s history of previous malignancy are particularly important to know. Histologically, the tumor cells should be assessed for cytologic features such as enlarged nuclei, prominent nucleoli and architectural findings such as a sheet, cord or tubular arrangement. Immunohistochemistry can be helpful; however, the pathologist needs to be aware of the sensitivity and specificity of the stains utilized. Many of these epithelioid-like sarcomas can be positive for specific cytokeratins, such as CK7. Of note, although historically utilized to identify mesenchymal differentiation, vimentin is no longer considered diagnostically useful in this respect, given its incredible lack of specificity.
The aim of this review is to provide an overview of sarcomas with epithelioid morphology that can be encountered in the mediastinum.
Adipocytic tumors
Dedifferentiated liposarcoma with epithelial-like cells
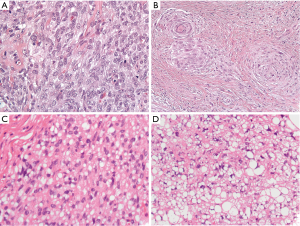
Well-differentiated liposarcoma typically arises in the deep soft tissue of the extremities or the retroperitoneum with approximately 2% presenting in the mediastinum (3). These low-grade sarcomas can grow quite large and typically exhibit some degree of adipocytic features which make classification relatively straightforward (4,5). They can occasionally, however, transition to higher grade dedifferentiated liposarcomas which no longer exhibit adipocytic features. Histologically, dedifferentiated liposarcomas are typically composed of highly atypical spindled cells which are arranged in a storiform architecture, sometimes emulating undifferentiated high-grade pleomorphic sarcoma (5). Rarely, these dedifferentiated liposarcomas have been found to have a spiraling arrangement of cells with associated bone or epithelioid-like cells that have been identified as meningothelial-like whorls. Sometimes these epithelioid-like cells can become prominent and extend beyond these meningothelial-like whorls in a nested or sheet like arrangement in the background of fibrous tissue (Figure 1A,B). Typically, dedifferentiated liposarcoma will have an associated well-differentiated liposarcoma component that greatly facilitates diagnosis. In a needle core biopsy or in tumors without a low-grade component, identification of MDM2 gene amplification by FISH can help confirm the diagnosis (4,6). Dedifferentiated liposarcomas will recur in 40% of cases and approximately 20% of these tumors metastasize (5).
Pleomorphic liposarcoma with epithelioid-like features
Pleomorphic liposarcoma is the least common variant of liposarcoma. Although a rare tumor in the mediastinum, it appears to have a predilection for this location relative to other sites (4,6-8). Histologically, it typically manifests as a high-grade sarcoma that contains at least a focal population of highly atypical cells which emulate the features of immature adipocytes (termed “lipoblasts”). Although these tumors are typically associated with a pleomorphic appearance, almost a third of pleomorphic liposarcomas can harbor more epithelioid-like cells which can mimic a renal cell carcinoma or adrenocortical carcinoma (Figure 1C,D). Of these tumors with epithelioid-like areas, nearly 40% will focally stain with a broad-spectrum cytokeratin (AE1/AE3) (9). Unlike other liposarcomas, they do not exhibit a characteristic genetic abnormality, such as MDM2 gene amplification. After excluding dedifferentiated liposarcoma, the diagnosis is primarily based on morphologic features. Clinically, they usually have a quick and frequent rate of metastatic spread (approximately 32%) (4).
Vascular tumors
Epithelioid angiosarcoma
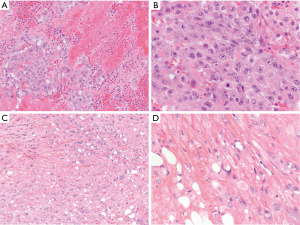
Angiosarcoma is an aggressive malignant vascular neoplasm that can arise in the anterior mediastinum in young to older adults, reportedly ranging from 3 to 12 cm in greatest dimension (10). They are rare, representing approximately 4% of angiosarcomas of the soft tissue (11). Histologically, malignant endothelial cells form ill-formed vascular like spaces that infiltrate soft tissue (11). In the epithelioid variant of angiosarcoma, a substantial portion of the malignant endothelial cells are arranged in diffuse sheets of epithelioid like cells with violet or amphophilic cytoplasm and vesicular nuclei (Figure 2A,B). Some pseudoglandular-like spaces can be seen. This particular variant of angiosarcoma has a predilection for deep soft tissue sites such as retroperitoneum, abdomen or thigh. These tumors have rarely been reported in the mediastinum (12-14). While these tumors will typically stain for vascular markers such as CD31, CD34 and ERG, a substantial portion of epithelioid angiosarcomas have been found to be positive for AE1/AE3 pancytokeratin (11,13,15,16). When making the diagnosis of epithelioid angiosarcoma, one should be careful to exclude other epithelioid vascular tumors such as epithelioid hemangioma or epithelioid hemangioendothelioma. These tumors exhibit more cytologic atypia than those found in an epithelioid hemangioma. The cells in an epithelioid hemangioendothelioma will exhibit a WWTR1-CAMTA1 fusion transcript by sequencing or nuclear expression of CAMTA1 by immunohistochemistry. Given the rarity of angiosarcomas in the mediastinum, there is limited documentation of the clinical behavior of these tumors in this specific location. Generally, however, these are highly aggressive soft tissue tumors with patients exhibiting a median survival of approximately one year (11).
Epithelioid hemangioendothelioma
Epithelioid hemangioendothelioma is another vascular tumor which is considered malignant but not as aggressive as angiosarcoma. Clinically, these tumors can vary in size and often arise as a well-circumscribed or locally infiltrative mass in the anterior mediastinum. True to their name, they are composed of varying amount of epithelioid-like endothelial cells with cytoplasmic vacuolization in a background of hyalinized fibrosis (Figure 2C,D). Sometimes, erythrocytes can be found in the vacuoles of the cells (17). Similar to epithelioid angiosarcoma, epithelioid hemangioendotheliomas will stain for endothelial markers such as CD31, CD34 and ERG. Immunohistochemical staining of CAMTA1 is considered to be relatively specific, consistent with the WWTR1-CAMTA1 fusion transcript associated with this tumor. The diagnosis of epithelioid hemangioendothelioma can sometimes be difficult since up to one third of these tumors can show focal staining for AE1/AE3 which can mislead a pathologist into making an erroneous diagnosis of metastatic carcinoma (13). When exhibiting higher grade cytologic features, epithelioid hemangioendothelioma can morphologically overlap with an angiosarcoma with epithelioid features. Epithelioid angiosarcomas, however, lack the WWTR1-CAMTA1 fusion transcript associated with this entity (13,18). Of epithelioid hemangioendotheliomas in the soft tissue, approximately 20% metastasize. Larger tumors with increased mitoses have a more aggressive clinical course (19). Although the data is limited, patients with epithelioid hemangioendotheliomas arising in the mediastinum have done well (17).
Peripheral nerve sheath tumors
Malignant peripheral nerve sheath tumor with divergent glandular differentiation
Malignant peripheral nerve sheath tumor is typically a high-grade malignant neoplasm that frequently arises in association with a neurofibroma or a peripheral nerve. Approximately half of these tumors will arise in patients with neurofibromatosis. (20). Microscopically, malignant peripheral nerve sheath tumor typically appears like a high-grade malignant spindle cell proliferation with areas of coagulative necrosis and numerous mitoses. Very rarely, malignant peripheral nerve sheath tumor can have heterologous components such as skeletal muscle, or clusters of glandular cells. In the latter entity, the glands lack cytologic atypia and can be columnar or cuboidal in appearance and often contain mucin. These glands typically are in the background of a more conventional malignant peripheral nerve sheath tumor with a spindled morphology (Figure 3). Immunohistochemically, the glandular elements in this tumor are positive for keratin and CEA and even express neuroendocrine markers such as synaptophysin (21,22).
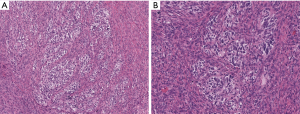
Malignant peripheral nerve sheath tumors with glandular differentiation are extraordinarily rare and most typically arise in the retroperitoneum. A case was recently reported in the mediastinum (21). The glandular elements are generally thought to be an incidental finding that does not change the generally grim prognosis of these tumors.
Other sarcomas with epithelioid morphology
Synovial sarcoma with prominent epithelial component
Synovial sarcomas are mesenchymal tumors that can have prominent and complex arrangements of epithelial-like cells. Despite the name, the tumor exhibits no credible features of synovial differentiation. Although commonly arising in the extremities, these tumors can arise in a broad diversity of locations, including the mediastinum (16,23). They occur over a broad age range of and can arise in either the anterior or posterior mediastinum, often growing quite large prior to identification and diagnosis (23,24). Histologically, synovial sarcomas can exhibit a broad range of morphologic features. Classically, there is an intimate admixture of both epithelioid-like and spindled cells that have varying amounts of associated collagen and sometimes calcifications. This is typically referred to as the biphasic type of synovial sarcoma. In the monophasic variant of synovial sarcoma, the tumor is exclusively composed of spindled cells with no obvious epithelial or glandular elements. Poorly differentiated synovial sarcomas emulate “round cell” sarcomas and typically exhibit substantial mitoses and necrosis (25). Occasionally, a synovial sarcoma can contain an overwhelming portion of epithelial-like cells which some have described as a monophasic epithelial-type synovial sarcoma or synovial sarcoma, epithelial/glandular variant. These epithelioid cells can be arranged in sheets, nests and even complex cribriform formations (Figure 4).
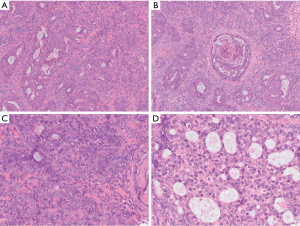
It is unclear whether a monophasic epithelial synovial sarcoma truly exists or if it simply represents a “epithelial-cell” predominant biphasic synovial sarcoma. Ultimately, this distinction has little clinical significance as long as pathologists and clinicians who treat the patient understand that the epithelial component of a synovial sarcoma can mimic entities such as carcinoma or mesothelioma when seen in the mediastinum. Synovial sarcoma expresses antigens frequently associated with carcinomas, including EMA and many cytokeratins, such as AE1/AE3, CK7, CK19, CK8/18, and high- and low-molecular weight cytokeratins (16,26). Over the past few years, a TLE-1 stain has been found to have high sensitivity for synovial sarcomas. However, this stain can also be positive in histologic mimickers of this tumor, including malignant peripheral nerve sheath tumor and solitary fibrous tumor. As almost all mesotheliomas are positive for TLE-1, this stain provides little utility for confirmation of synovial sarcoma in the mediastinum. Recently reported SS18-SSX fusion specific antibodies could potentially provide more specific clinically available stains for synovial sarcoma in the near future (27). Synovial sarcomas harbor a distinct SS18-SSX1 or SS18-SSX2 gene fusion that can be detected by FISH, RT-PCR or next generation sequencing (25). Primary pulmonary and mediastinal synovial sarcomas have been observed to have more aggressive behavior when compared to tumors in other soft tissue sites (28). Of patients with synovial sarcoma of the mediastinum, approximately 67% had disease progression with a median time to progression of 18 months. Complete excision is associated with increased overall survival (23).
SMARCA4-deficient thoracic sarcoma
The SMARCA4 gene is responsible for the BRG1 protein which forms one of the subunits of the switch/sucrose-nonfermenting chromatin-remodeling complex (SWI/SNF). Inactivation of SMARCA4 has recently been described in various malignancies with a “rhabdoid” morphology, including small cell carcinoma of the ovary and a subset of atypical teratoid rhabdoid tumors found in the central nervous system (29). Recently, similar SMARCA4 inactivation was identified in a group of undifferentiated thoracic malignancies but were felt to be similar to other sarcomas with similar histologic and immunohistochemical features (29-31). These aggressive thoracic tumors often involve the mediastinum. Typically, they are quite large, precluding resection. Although initially described as arising in younger adults, these tumors can arise in a broad age range of patients spanning between the second to eighth decade of life (30). Metastasis (to sites such as the lymph nodes, bone and adrenal glands) are typically present at the time of diagnosis (29,32). Clinically, these tumors are extraordinarily aggressive with patient’s typically expiring within one year of diagnosis (31). Histologically, the tumor contains sheets and nests of syncytial or discohesive epithelioid to round cells with mildly eosinophilic to amphophilic cytoplasm. Focal areas of tumor cells have more monomorphic morphology with round and vesicular nuclei and prominent nucleoli. Rhabdoid cells, which have more abundant and glassy eosinophilic cytoplasm, can be usually be focally or rarely seen. Substantial mitoses and abundant necrosis are present (Figure 5) (6,30). Immunohistochemically, these tumors typically show at focal weak to moderate AE1/AE3 expression. The tumor cells can also stain for EMA, CD34, SALL4 and SOX2. While SMARCB1 is typically retained, SMARCA4 and SMARCA2 are lost in the tumor cells. The tumor cells are negative for NUT, S100 and desmin staining (30).
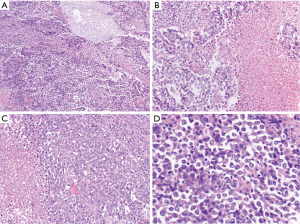
Although historically designated as sarcomas, the true nature of these SMARCA4-deficient tumors has been a topic of enthusiastic discussion. In a recent paper by Rekhtman et al., the authors identified SMARCA4-deficient tumors which contained an associated component of non-small cell lung carcinoma or focal expression of TTF-1 or p40. From these and other findings, it was proposed these SMARCA4-deficient thoracic sarcomatoid tumors actually represent undifferentiated or dedifferentiated carcinomas rather than primary sarcomas (32). As the clinical and molecular characteristics of these tumors becomes better understood, this will continue to be a subject of intense interest.
Alveolar soft part sarcoma
Alveolar soft part sarcoma is a soft tissue neoplasm that frequently arises in the proximal extremity in adolescents or young adults. In infants and children, it is more frequently associated with the head and neck area (33-35). There are also reports of less common anatomic sites, such as the genitourinary tract (36-39). There are only a handful of reports documenting alveolar soft part sarcoma arising in the mediastinum (40,41). Typically, these tumors are slow growing and can be quite large by the time they are identified. A substantial percentage of patients (20–70%) will have metastatic disease (e.g., lung, brain and bone) (42).
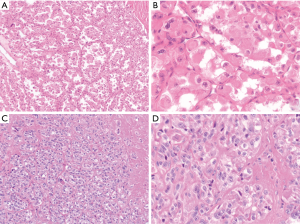
Histologically, these tumors display a consistent monomorphic population of epithelioid cells arranged in a trabecular, nested or alveolar architecture. The cells typically contain eosinophilic cytoplasm but they can exhibit focal clearing. The nuclei are vesicular and often contain prominent nucleoli (Figure 6A,B) (38). Immunohistochemically, the tumor cells are usually positive for TFE3 and CD68 and are generally negative for cytokeratins, EMA, S100 and HMB45. A Periodic acid-Schiff (PAS) stain can demonstrate rhomboid or rod-shaped crystals in the cytoplasm of the tumor cells (43). Next generation sequencing, RT-PCR or FISH studies can be utilized to demonstrate the characteristic ASPL-TFE3 fusion transcript associated with this tumor (44). Given the epithelioid nature of alveolar soft part sarcomas, the differential diagnosis frequently includes metastatic carcinoma or melanoma. The lack of keratin or S100 staining helps excludes these two possibilities, respectively. Of note, certain types of renal cell carcinoma will be positive for TFE3; however, alveolar soft part sarcomas will typically lack PAX8 staining associated with these tumors (45). PEComas have been demonstrated to stain for TFE3 by immunohistochemistry; however, they will not exhibit the ASPL-TFE3 fusion transcript associated with alveolar soft part sarcoma. Although alveolar soft part sarcomas are slow growing tumors, they have a long-term clinically aggressive course. While survival in patients without metastasis at initial presentation was 77% at 2 years, it was only 38% and 15% at 10 year and 20 years, respectively (46). The tumors are typically resistant to chemotherapy and radiation therapy and local control with adequate surgical excision is the mainstay of treatment (33).
Clear cell sarcoma of soft tissue
Clear cell sarcoma of soft tissue is a tumor which most commonly arises in the distal extremities in association with a tendon or aponeurosis in young adults (previously designed as clear cell sarcoma of the tendon sheath). While these tumors typically show a prominent spindled morphology, some tumors can exhibit a predominantly epithelioid appearance. The tumors frequently have an indolent presentation and can be clinically present many years before histologic diagnosis (47,48). A handful of these tumors have been reported as large masses arising in the mediastinum with the tumor cells exhibiting epithelioid features (13,49,50). Histologically, clear cell sarcomas are characterized by epithelioid to spindled cells with eosinophilic or clear cytoplasm. The nuclei are enlarged and contain prominent nucleoli. The cells are typically in the background of fibrotic stroma with dense fibrosis. Multinucleated tumor giant cells and melanin pigment deposition can be seen (Figure 6C,D) (16,48). Immunohistochemically, these tumors have a similar staining pattern to melanomas, with the tumor cells being positive for S100, SOX10, HMB-45 and Melan A. Unlike melanomas, however, these tumors exhibit an EWSR1-ATF1 fusion transcript. Interestingly EWSR1-ATF1 is not specific for this tumor, as this fusion has been identified in completely unrelated entities such as angiomatoid fibrous histiocytoma, hyalinizing clear cell carcinoma of the salivary gland and primary pulmonary myxoid sarcoma (51). The diversity of tumors harboring a similar fusion transcript emphasizes the importance of a holistic diagnostic approach when investigating a soft tissue sarcoma. In addition to melanoma, the morphologic differential includes other entities which can include epithelioid to spindled cells such as synovial sarcoma or perhaps malignant peripheral nerve sheath tumor. The presence of strong S100, HMB45 and Melan-A staining (with the concurrent presence of EWSR-ATF1 fusion transcript) easily distinguishes clear cell sarcoma of soft tissue from these histological mimickers. The limited occurrence of clear cell sarcoma in the mediastinum precludes site specific assessment of prognosis. Generally, although clear cell sarcoma typically has an indolent presentation, the long-term clinical outcome is poor. While the 5-year survival rate is 67%, the 20-year survival rate is 10%. Increased tumor size is associated with a poorer prognosis (48).
Conclusions
In the mediastinum, the diagnosis of soft tissue sarcomas with epithelioid morphology can be a difficult endeavor. Not only do these tumors morphologically emulate the features of carcinoma or mesothelioma more frequently found in this location, but they also frequently stain for EMA and cytokeratin stains typically associated with epithelial neoplasms. As sarcomas and carcinoma typically have unique adjuvant treatment regimens, the diagnosis is clinically relevant. A holistic diagnostic approach and familiarity with the specific morphologic, immunohistochemical and molecular findings associated with these soft tissue neoplasms can help assure accurate diagnosis.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Saul Suster and David Suster) for the series “Mediastinal Sarcomas” published in Mediastinum. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-61). The series “Mediastinal Sarcomas” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dubashi B, Cyriac S, Tenali SG. Clinicopathological analysis and outcome of primary mediastinal malignancies - A report of 91 cases from a single institute. Ann Thorac Med 2009;4:140-2. [Crossref] [PubMed]
- Szolkowska M, Szczepulska-Wojcik E, Maksymiuk B, et al. Primary mediastinal neoplasms: a report of 1,005 cases from a single institution. J Thorac Dis 2019;11:2498-511. [Crossref] [PubMed]
- Krishnasamy S, Krishna Nair A, Hashim SA, et al. Mediastinal liposarcoma: a rare visceral mediastinal tumour. Interact Cardiovasc Thorac Surg 2019;29:976-7. [Crossref] [PubMed]
- Hahn HP, Fletcher CD. Primary mediastinal liposarcoma: clinicopathologic analysis of 24 cases. Am J Surg Pathol 2007;31:1868-74. [Crossref] [PubMed]
- Ortega P, Suster D, Falconieri G, et al. Liposarcomas of the posterior mediastinum: clinicopathologic study of 18 cases. Mod Pathol 2015;28:721-31. [Crossref] [PubMed]
- Boland JM, Colby TV, Folpe AL. Liposarcomas of the mediastinum and thorax: a clinicopathologic and molecular cytogenetic study of 24 cases, emphasizing unusual and diverse histologic features. Am J Surg Pathol 2012;36:1395-403. [Crossref] [PubMed]
- Barbetakis N, Asteriou C, Kleontas A, et al. Primary pleomorphic liposarcoma: a rare mediastinal tumor. Interact Cardiovasc Thorac Surg 2010;11:327. [Crossref] [PubMed]
- Romero-Guadarrama MB, Jimenez-Becerra S, Duran-Padilla MA, et al. Mediastinal pleomorphic liposarcoma diagnosed by fine needle aspiration biopsy: a case report. Acta Cytol 2007;51:440-2. [Crossref] [PubMed]
- Hornick JL, Bosenberg MW, Mentzel T, et al. Pleomorphic liposarcoma: clinicopathologic analysis of 57 cases. Am J Surg Pathol 2004;28:1257-67. [Crossref] [PubMed]
- Weissferdt A, Kalhor N, Suster S, et al. Primary angiosarcomas of the anterior mediastinum: a clinicopathologic and immunohistochemical study of 9 cases. Hum Pathol 2010;41:1711-7. [Crossref] [PubMed]
- Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol 1998;22:683-97. [Crossref] [PubMed]
- Demiröz ŞM, Fındık G, Aydoğdu K, et al. Mediastinal epithelioid angiosarcoma arising in schwannoma: The first case in the literature. Turk Gogus Kalp Damar Cerrahisi Derg 2018;26:305-8. [Crossref] [PubMed]
- Anderson T, Zhang L, Hameed M, et al. Thoracic epithelioid malignant vascular tumors: a clinicopathologic study of 52 cases with emphasis on pathologic grading and molecular studies of WWTR1-CAMTA1 fusions. Am J Surg Pathol 2015;39:132-9. [Crossref] [PubMed]
- Tane S, Tanaka Y, Tauchi S, et al. Radically resected epithelioid angiosarcoma that originated in the mediastinum. Gen Thorac Cardiovasc Surg 2011;59:503-6. [Crossref] [PubMed]
- Fletcher CD, Beham A, Bekir S, et al. Epithelioid angiosarcoma of deep soft tissue: a distinctive tumor readily mistaken for an epithelial neoplasm. Am J Surg Pathol 1991;15:915-24. [Crossref] [PubMed]
- Goldblum JR, Enzinger FM, Folpe AL, et al. editors. Enzinger and Weiss's soft tissue tumors. Philadelphia, PA: Elsevier; 2014.
- Suster S, Moran CA, Koss MN. Epithelioid hemangioendothelioma of the anterior mediastinum. Clinicopathologic, immunohistochemical, and ultrastructural analysis of 12 cases. Am J Surg Pathol 1994;18:871-81. [Crossref] [PubMed]
- Doyle LA, Fletcher CD, Hornick JL. Nuclear expression of CAMTA1 distinguishes epithelioid hemangioendothelioma from histologic mimics. Am J Surg Pathol 2016;40:94-102. [Crossref] [PubMed]
- Deyrup AT, Tighiouart M, Montag AG, et al. Epithelioid hemangioendothelioma of soft tissue: a proposal for risk stratification based on 49 cases. Am J Surg Pathol 2008;32:924-7. [Crossref] [PubMed]
- James AW, Shurell E, Singh A, et al. Malignant peripheral nerve sheath tumor. Surg Oncol Clin N Am 2016;25:789-802. [Crossref] [PubMed]
- Thway K, Hamarneh W, Miah AB, et al. Malignant peripheral nerve sheath tumor with rhabdomyosarcomatous and glandular elements: rare epithelial differentiation in a Triton tumor. Int J Surg Pathol 2015;23:377-83. [Crossref] [PubMed]
- Woodruff JM. Peripheral nerve tumors showing glandular differentiation (glandular schwannomas). Cancer 1976;37:2399-413. [Crossref] [PubMed]
- Salah S, Salem A. Primary synovial sarcomas of the mediastinum: a systematic review and pooled analysis of the published literature. ISRN Oncol 2014;2014:412527. [Crossref] [PubMed]
- Folpe AL, Schmidt RA, Chapman D, et al. Poorly differentiated synovial sarcoma: immunohistochemical distinction from primitive neuroectodermal tumors and high-grade malignant peripheral nerve sheath tumors. Am J Surg Pathol 1998;22:673-82. [Crossref] [PubMed]
- Thway K, Fisher C. Synovial sarcoma: defining features and diagnostic evolution. Ann Diagn Pathol 2014;18:369-80. [Crossref] [PubMed]
- Doyle VJ, Bateman AC, Theaker JM. An unusual breast mass: primary synovial sarcoma. BMJ Case Rep 2013;2013:bcr2013010468. [Crossref] [PubMed]
- Baranov E, McBride MJ, Bellizzi AM, et al. A novel SS18-SSX fusion-specific antibody for the diagnosis of synovial sarcoma. Am J Surg Pathol 2020;44:922-33. [Crossref] [PubMed]
- Hartel PH, Fanburg-Smith JC, Frazier AA, et al. Primary pulmonary and mediastinal synovial sarcoma: a clinicopathologic study of 60 cases and comparison with five prior series. Mod Pathol 2007;20:760-9. [Crossref] [PubMed]
- Sauter JL, Graham RP, Larsen BT, et al. SMARCA4-deficient thoracic sarcoma: a distinctive clinicopathological entity with undifferentiated rhabdoid morphology and aggressive behavior. Mod Pathol 2017;30:1422-32. [Crossref] [PubMed]
- Yoshida A, Kobayashi E, Kubo T, et al. Clinicopathological and molecular characterization of SMARCA4-deficient thoracic sarcomas with comparison to potentially related entities. Mod Pathol 2017;30:797-809. [Crossref] [PubMed]
- Le Loarer F, Watson S, Pierron G, et al. SMARCA4 inactivation defines a group of undifferentiated thoracic malignancies transcriptionally related to BAF-deficient sarcomas. Nat Genet 2015;47:1200-5. [Crossref] [PubMed]
- Rekhtman N, Montecalvo J, Chang JC, et al. SMARCA4-deficient thoracic sarcomatoid tumors represent primarily smoking-related undifferentiated carcinomas rather than primary thoracic sarcomas. J Thorac Oncol 2020;15:231-47. [Crossref] [PubMed]
- Orbach D, Brennan B, Casanova M, et al. Paediatric and adolescent alveolar soft part sarcoma: A joint series from European cooperative groups. Pediatr Blood Cancer 2013;60:1826-32. [Crossref] [PubMed]
- Pennacchioli E, Fiore M, Collini P, et al. Alveolar soft part sarcoma: clinical presentation, treatment, and outcome in a series of 33 patients at a single institution. Ann Surg Oncol 2010;17:3229-33. [Crossref] [PubMed]
- Wang HW, Qin XJ, Yang WJ, et al. Alveolar soft part sarcoma of the oral and maxillofacial region: clinical analysis in a series of 18 patients. Oral Surg Oral Med Oral Pathol Oral Radiol 2015;119:396-401. [Crossref] [PubMed]
- Feng M, Jiang W, He Y, et al. Primary alveolar soft part sarcoma of the uterine cervix: a case report and literature review. Int J Clin Exp Pathol 2014;7:8223-6. [PubMed]
- Kang WD, Heo SH, Choi YD, et al. Alveolar soft part sarcoma of the uterine cervix in a woman presenting with postmenopausal bleeding: a case report and literature review. Eur J Gynaecol Oncol 2011;32:359-61. [PubMed]
- Lee HJ. Alveolar soft part sarcoma of the uterine cervix: a case report and review of the literature. Korean J Pathol 2014;48:361-5. [Crossref] [PubMed]
- Ryu A, Mun ST, Lee HJ, et al. Recurrent alveolar soft part sarcoma of the uterine cervix. J Obstet Gynaecol 2017;37:1099-101. [Crossref] [PubMed]
- Flieder DB, Moran CA, Suster S. Primary alveolar soft-part sarcoma of the mediastinum: a clinicopathological and immunohistochemical study of two cases. Histopathology 1997;31:469-73. [Crossref] [PubMed]
- Kameda Y, Nishii T, Tsuboi M, et al. Alveolar soft-part sarcoma of the mediastinum: A case report. SAGE Open Med Case Rep 2017;5:2050313X17695473.
- Schenning R, Vajtai P, Troxell M, et al. Alveolar soft part sarcoma: unusual etiology of mediastinal mass in an adolescent. Clin Pract 2013;3:e26. [Crossref] [PubMed]
- Zhang H, Wang Y, Liu Y, et al. Alveolar soft part sarcoma of uterine cervix in a postmenopausal woman: a case report and review of literature. Int J Clin Exp Pathol 2017;10:9812-5. [PubMed]
- Williams A, Bartle G, Sumathi VP, et al. Detection of ASPL/TFE3 fusion transcripts and the TFE3 antigen in formalin-fixed, paraffin-embedded tissue in a series of 18 cases of alveolar soft part sarcoma: useful diagnostic tools in cases with unusual histological features. Virchows Arch 2011;458:291-300. [Crossref] [PubMed]
- Rao Q, Williamson SR, Zhang S, et al. TFE3 break-apart FISH has a higher sensitivity for Xp11.2 translocation-associated renal cell carcinoma compared with TFE3 or cathepsin K immunohistochemical staining alone: expanding the morphologic spectrum. Am J Surg Pathol 2013;37:804-15. [Crossref] [PubMed]
- Lieberman PH, Brennan MF, Kimmel M, et al. Alveolar soft-part sarcoma. A clinico-pathologic study of half a century. Cancer. 1989;63:1-13. [Crossref] [PubMed]
- Hornick JL. Practical soft tissue pathology: a diagnostic approach. Philadelphia, PA: Elsevier, 2019.
- Lucas DR, Nascimento AG, Sim FH. Clear cell sarcoma of soft tissues. Mayo Clinic experience with 35 cases. Am J Surg Pathol 1992;16:1197-204. [Crossref] [PubMed]
- Tanaka Y, Yoshimasu T, Oura S, et al. Primary clear-cell sarcoma in the mediastinum. Case Rep Oncol 2014;7:306-9. [Crossref] [PubMed]
- Tirabosco R, Lang-Lazdunski L, Diss TC, et al. Clear cell sarcoma of the mediastinum. Ann Diagn Pathol 2009;13:197-200. [Crossref] [PubMed]
- Thway K, Fisher C. Tumors with EWSR1-CREB1 and EWSR1-ATF1 fusions: the current status. Am J Surg Pathol 2012;36:e1-11. [Crossref] [PubMed]
Cite this article as: Perry KD, Montecalvo J, Perry AM. Sarcomas of the mediastinum with epithelioid morphology. Mediastinum 2021;5:4.


