Role of endobronchial ultrasound-guided transbronchial needle aspiration in staging of lung cancer: a thoracic surgeon’s perspective
Introduction
Lung cancer is the leading cause of cancer deaths accounting for nearly 1.59 million deaths annually worldwide. Approximately 43% of patients with lung cancer are diagnosed with metastatic spread upon initial presentation (1).
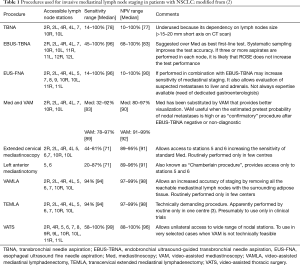
In potentially resectable non-small cell lung cancer (NSCLC) accurate mediastinal staging is crucial not only to offer the optimal management but also to avoid unnecessary surgery. The sampling of mediastinal lymph nodes can be performed using several procedures (Table 1).
Full table
Mediastinoscopy was first introduced by Carlens (4) in the early sixties and for a long period it was considered the gold standard for pre-surgical mediastinal nodal staging of NSCLC. Since mediastinoscopy cannot access pre-vascular (station 3A), sub-aortic (station 5) and para-aortic (station 6) nodes, extended cervical mediastinoscopy and left anterior mediastinotomy (Chamberlain procedure) have been developed as staging procedures to be used in carefully selected patients.
More recently mediastinoscopy has been replaced by video-assisted mediastinoscopy, increasing diagnostic yield and procedure safety (5,6). The use of video techniques has significantly improved the procedure performance, allowing visualization of magnified images on the monitor and their simultaneous sharing with all personnel in the operating theatre (7,8). These technological advances were essential for the development of two new mediastinal staging surgical procedures (9): the video-assisted mediastinal lymphadenectomy (VAMLA), exploring the right and left paratracheal and subcarinal nodes, and the transcervical extended mediastinal lymphadenectomy (TEMLA), exploring all mediastinal node stations from supraclavicular to paraesophageal. Although the reported sensitivity and negative predictive value (NPV) of VAMLA and TEMLA were high, these techniques have demonstrated a limited clinical diffusion due to their technical difficulties and a relative high rate of complications (6–13.2%) (3,9-11).
In the last 20 years we have assisted to a progressive introduction of endoscopic needle-based techniques. The first endoscopic procedure used for preoperative mediastinal staging was the transbronchial needle aspiration (TBNA), a “blind” minimally invasive method for sampling lymph nodes adjacent to the tracheobronchial wall. Although numerous studies have demonstrated that TBNA has a high sensibility, is safe and is cost-effective comparable to mediastinoscopy, only a minority of pulmonologists and thoracic surgeons have used this endoscopic procedure for preoperative mediastinal staging of NSCLC (6,12,13). The main reason for its underuse was the belief that this technique had an adequate accuracy only in patients with significantly enlarged (mainly in the subcarinal region) lymph nodes (>2 cm) (6,12).
In recent years, endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) has progressively replaced conventional “blind” TBNA as test for invasive mediastinal staging. The considerable potential of EBUS-TBNA as minimally invasive staging method has been understood by the pulmonologists since the early 2000s but only recently by the thoracic surgeons. Being the clinical impact of this endoscopic technology broadly highlighted in the literature, EBUS-TBNA is universally considered the first-choice procedure for invasive mediastinal nodal staging of NSCLC patients (2).
EBUS-TBNA, as well as mediastinoscopy, does not allow access to all mediastinal lymph node stations. To overcome this limitation a different needle-based technique has been used in preoperative staging of NSCLC—the endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA)—alone or in association with EBUS-TBNA.
The combined use of EBUS-TBNA and EUS-FNA (combined endoscopic ultrasonography) seems to be associated to higher sensitivity and specificity for NSCLC mediastinal staging than those of EBUS-TBNA or EUS-FNA alone (5-8).
Endoscopic mediastinal staging with EBUS-TBNA and EUS-FNA
No single endoscopic mediastinal staging method allows access to all mediastinal lymph node stations (Table 1).
EBUS-TBNA can reach mediastinal stations 2R, 2L, 4R, 4L, and 7. Also the posterior area of station 7, not always easily accessible to videomediastinoscopy, can be reached with EBUS-TBNA (14,15), resulting in a more extensive evaluation of the subcarinal region. EBUS-TBNA allows also access to stations 10, 11 and 12. The possibility to sample also tissue from N1 lymph nodes may be very important in selected patients with multiple N1 nodal disease in whom a neoadjuvant therapy (although not currently standard of care) might have a rationale to increase the chances of achieving complete surgical resection and reduce the risk of disease recurrence.
EBUS-TBNA is a safe procedure that can be performed with a low incidence of minor complications (1.23%) in an endoscopic suite under conscious sedation. Reported complications include: mediastinitis, pneumonia, pericarditis, sepsis, pneumothorax and haemorrhage. EBUS-TBNA related mortality is 0.01% (8,16,17).
In two of the first studies published in the literature EBUS-TBNA showed a high diagnostic performance with a sensitivity, specificity, positive predictive value (PPV), NPV, and accuracy of 94.5%, 100%, 100%, 89.5% and 96.3% (18), and with a sensitivity, specificity, diagnostic yield and accuracy of 94%, 100%, 93% and 94% (19), respectively. Four systematic reviews and meta-analyses (20-23) subsequently confirmed its excellent diagnostic performance in NSCLC mediastinal staging. In the meta-analysis by Gu et al. (21) involving 1,299 patients the pooled sensitivity of EBUS-TBNA in mediastinal staging for lung cancer was 93% (95% CI, 91–94%).
EBUS-TBNA diagnostic performance in invasive mediastinal staging of NSCLC has been compared to the one of mediastinoscopy. In three prospective randomized studies (14,15,24) including a total of 346 patients the sensitivity ranged from 81% to 88% for EBUS-TBNA and from 68% to 81% for videomediastinoscopy, the NPV from 78% to 91% and from 59% to 90%, respectively. In these studies, videomediastinoscopy was associated with more complications than EBUS-TBNA. Moreover, contrarily to videomediastinoscopy EBUS-TBNA showed only minor and spontaneously resolved complications.
In the analysis of the performance data of EBUS-TBNA reported in the literature some considerations are necessary.
Despite the technological progress and the availability of new diagnostic procedures, such as EBUS TBNA and EUS-FNA, no clinical algorithm can eliminate the probability to discover a malignant mediastinal nodal involvement at surgical exploration. In this context, the real purpose in our daily clinical practice is to reduce the risk to the minimum (25).
Another goal of all thoracic surgeons and pulmonologists is to rule out, as best as they can, a N2/3 disease by appropriate use of the available diagnostic procedures (25). To do this correctly it is important to evaluate the diagnostic performance of EBUS-TBNA in mediastinal staging in the context of disease prevalence. In a low-prevalence patient population (cN0/N1 NSCLC) the reported sensitivity of this procedure is lower than that in a high-prevalence setting (cN2/3 NSCLC). Szlubowski et al. (26) and Dooms et al. (27) observed a sensitivity of 46% and 39% in patient populations with a nodal disease prevalence of 23% and 24%, respectively. In higher disease prevalence settings (77% and 80%, respectively), EBUS-TBNA increased the sensitivity to 91% and 97%, respectively (28,29).
Similarly to the preoperative surgical staging and the intraoperative pathologic staging, the endoscopic mediastinal staging performed with EBUS-TBNA may be systematic (sampling of all accessible nodes performing 3 or more passes per node) or selective (sampling of abnormal nodes). However, these technical aspects of EBUS-TBNA are not well considered in the literature (30) and few studies report in detail how many lymph nodes were biopsied and the number of passes for each node. An increase of sensitivity of EBUS-TBNA in mediastinal staging of NSCLC was noted when performing a systematic sampling over a more limited or selective approach (30,31). Some studies have demonstrated an increase of the diagnostic performance of EBUS-TBNA with a higher number of aspirations per node (32,33). Detterbeck et al. (30) have proposed for the most thorough assessment of the mediastinum three aspirates per node have to be made. On the other hand, some prospective studies (15,34) have demonstrated that the use of videomediastinoscopy in a low disease prevalence setting may not improve the staging sensitivity after a well-performed negative EBUS-TBNA with biopsy of at least three lymph node stations. These data stress the importance of the thoroughness of execution of EBUS-TBNA, not only to increase its diagnostic performance, but also to allow a better comparison of the results between the studies.
ROSE is a rapid, on-site evaluation of the tissue obtained with EBUS-TBNA allowing a proper specimen examination with an increased diagnostic performance. ROSE is considered an important adjunct technique to mediastinal staging of NSCLC. Using EBUS-TBNA, ROSE provides better sampling and a control on the cellularity of the specimen useful, for example, in highly necrotic lymph nodes (35). However, according to Detterbeck et al. (30) the use of ROSE can be omitted when performing three or more passes in each nodal station because it is likely that in this context this technique doesn’t increase the EBUS-TBNA sensitivity.
The quality of EBUS-TBNA depends on experience and skills of the operator (thoracic surgeon or interventional pulmonologist). The training methodology includes traditional training methods and simulator-based trainings. As reported by Sehgal et al. (36) to achieve an accuracy of at least 80%, 37–44 procedures are needed. The simulation-based training seems to be superior to the traditional apprenticeship models and it is recommended in the newest guidelines (37). The operator’s experience and skill are other factors that should be considered when analysing the performance data of EBUS-TBNA in mediastinal nodal staging of NSCLC.
EUS-FNA is an endoscopic procedure that can be performed with conscious sedation allowing biopsies of stations 5, 6, 8 and 9 in addition to the 2R, 2L, 4R, 4L, 7, 10R and 10L lymph nodes accessible by EBUS-TBNA (Table 1). Although stations 5 and 6 can be well visualized by EUS, they can rarely be sampled without traversing the pulmonary artery and/or the aorta (38). Stations 2R and 4R are difficult to biopsy by EUS-FNA because the trachea lies between the bronchoscope and the lymph nodes limiting the exploration of this region (38). In high-volume endosonography centres only occasional minor complications are reported after EUS-FNA (5,6).
In the vast majority of the studies reported in the literature, EUS-FNA diagnostic performances are very similar to that of EBUS-TBNA. In a systematic review (5) analyzing data of 2,433 patients undergoing mediastinal nodal staging of NSCLC with EUS-FNA a median sensitivity of 89% and a NPV of 86% were noted. A meta-analysis of EUS-FNA (6) reported a pooled sensitivity of 83%, with values ranging between 78 and 87%. In the meta-analysis by Micames et al. (39) the pooled sensitivity of EUS-FNA was 83% (95% CI, 78–87%). In this study the sensitivity was 90% (95% CI, 84–94%) among patients with enlarged mediastinal lymph nodes at CT scan and 58% (95% CI, 39–75%) in a subgroup of patients with a normal mediastinum.
Data from the literature show that the integration of EBUS-TBNA and EUS-FNA in a single endoscopic procedure (combined ultrasonography) to staging the mediastinum further increases the accuracy if compared with either technique alone (40,41). A meta-analysis conducted by Dhooria et al. (42) (4 studies and 465 patients) showed a pooled sensitivity of EBUS-TBNA alone of 80% (95% CI, 74–86%) whereas that of combined endosonography was 91% (95% CI, 86–95%).
Contemporary clinical practice guidelines for preoperative mediastinal lymph node staging for NSCLC
In 2013 both the American College of Chest Physicians (ACCP) (5) and the European Society of Thoracic Surgeons (ESTS) (6) developed guidelines for invasive mediastinal staging of NSCLC. More recently the European Society of Gastrointestinal Endoscopy (ESGE) published guidelines for the diagnosis and staging of lung cancer using combined endosonography (38).
According to the ESTS guidelines (6) in case of enlarged mediastinal lymph nodes on CT and/or PET-positive lymph nodes (cIIIA), endosonography (EBUS-TBNA or EUS FNA) is recommended over mediastinoscopy as the initial procedure. In the same patient population ESGE guidelines (38) recommend the use of combined endosonography with EBUS-TBNA plus EUS-FNA. However, if combined endosonography is not available, EBUS-TBNA alone may be acceptable. Both ESTS (6) and ESGE (38) guidelines recommend that a confirmatory mediastinoscopy is indicated, if the endoscopic staging is negative, In case of normal mediastinal lymph nodes on CT and PET-negative mediastinal lymph nodes but suspected N1 lymph nodes and/or central tumour and or tumour >3 cm, EBUS-TBNA or EUS-FNA or videomediastinoscopy (ESTS) or EBUS-TBNA plus EUS-FNA (ESGE) are recommended (6,38). In fact, about 25% of patients with these clinical characteristics have at resection an occult N2 disease (43). According to the ESTS guidelines the choice between endoscopic or surgical mediastinal exploration depends on local expertise to adhere to minimal requirements for staging (6). Assuming the ability to perform a thorough mediastinoscopy, the choice of an endoscopic method is based on the availability of skilled endoscopists able to perform a thorough procedure also in patients with small or normal sized mediastinal lymph nodes. If the endoscopic staging is negative, both ESTS and ESGE guidelines recommend to proceed directly to surgery without a confirmatory mediastinoscopy (6,38).
Both ESTS and ESGE guidelines agree on the possibility to avoid an invasive mediastinal staging in patients without suspected lymph nodes detected by CT and/or PET and with a tumour ≤3 cm and located in the outer third of the lung (Table 2).

Full table
Despite the current guidelines (5,6,38) are supported by high levels of evidence, there continues to be significant variability and underuse of invasive mediastinal staging of NSCLC (2,44-48). A significant number of stage IIIA patients do not receive guideline-adherent mediastinal staging. Among those NSCLC patients who received invasive mediastinal staging the majority underwent mediastinoscopy and only a small percentage (15%) EBUS-TBNA (44). Low rates of EBUS-TBNA and mediastinoscopy may be considered a reflection of a poor-quality care (2). It is likely that thoracic surgeons do not adhere to guidelines because they do not accept the recommendations to be valid or they believe the supporting evidence cannot be generalizable to their clinical practice (44).
EBUS-TBNA and EUS-FNA for re-staging after neo-adjuvant therapy for stage IIIA NSCLC
Currently, no imaging technique can accurately determine the biological response of the IIIA NSCLC to the neo-adjuvant treatment. The efficacy of CT scan in restaging the mediastinum is low, with a sensitivity of 50%, a specificity of 65% and an accuracy of 60% (49,50). In the mediastinal restaging after induction therapy PET scan is less sensitive than in primary staging with a sensitivity ranging between 50% and 60%. Although PET-CT fusion images increase the accuracy as compared to CT and PET alone for restaging (7,49), to identify patients who can benefit from lung resection after neo-adjuvant therapy, an invasive technique providing cytohistological information may be necessary. For many thoracic surgeons, in fact, mediastinal nodal downstaging is a very important prognostic factor for long-term survival and therefore a parameter to select patients for surgery (51).
The same invasive methods for primary staging can be used to assess the mediastinum status after neo-adjuvant therapy for stage IIIA NSCLC (51). In this setting, however, both EBUS-TBNA and videomediastinoscopy have shown worse performance capacities than in primary staging. Main published experiences (7,26,49,52,53) report a sensitivity ranging from 67% to 76% and a NPV ranging from 20% to 78% for EBUS-TBNA and a sensitivity ranging from 29% to 74% and a NPV ranging from 52% to 74% for re-mediastinoscopy, respectively. This is mainly due to mediastinal scarring secondary to induction therapy and prior mediastinoscopy (7,8). Although re-mediastinoscopy is technically feasible, it is associated not only to a lower accuracy but also to an increased risk of major complications compared with mediastinoscopy for primary staging.
Therefore, in this clinical scenario a possible strategy could be to use EBUS-TBNA for baseline mediastinal staging and a first videomediastinoscopy or a second endosonography for restaging after neoadjuvant chemotherapy.
Our NSCLC preoperative mediastinal staging workflow
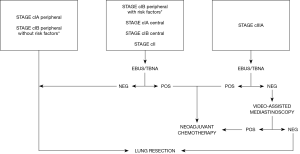
In our clinical practice the indications to perform invasive mediastinal staging before a planned resection of a NSCLC vary in accordance with the clinical scenario as determined by CT and PET scan (Figure 1).
Clinical stage IA (peripheral T1abcN0)
In clinical stage I NSCLC the NPV of PET/CT for occult mediastinal nodal metastases is 94% (54). In the studies by Cerfolio et al. (55) and Meyers et al. (56) in clinical stage I NSCLC patients a postsurgical upstaging to pathologic N2 (pN2) disease was noted in 2.75 and 3%, respectively. In this context a negative mediastinal PET/CT seems to be highly predictive of node-negative mediastinum and probably does not require cytohistological confirmation. In this clinical scenario we think that to omit preoperative invasive mediastinal staging proceeding directly to lung resection is a reasonable choice because the probability of an occult N2 disease is very low. Although no clear definition of low-probability is reported in the literature, ESTS guidelines (6) consider as acceptable a rate of unforeseen N2 disease at surgery up to 10% (25). It is important to note that in this subset of patients with small (not more than 3 cm in larger diameter) and peripheral (located in the outer third of the lung) lung tumour, mediastinal involvement revealed by resection is usually represented by single N2 stations (57) with an excellent long-term survival. In the series by Kim et al. (58) the 5-year survival rate for occult stage IIIA-N2 was 48% significantly better than that reported for stage IIIA-N2 in the 7th edition of AJCC staging for NSCLC (24%) (59).
Clinical stage IB (peripheral T2aN0)
Although the pre-test probability of N2 metastases in clinical stage IB is higher than that in clinical stage IA, it appears to be still sufficiently low. As reported by Wang et al. (54) occult mediastinal nodal metastases are present in 11% of cT2N0 NSCLC after PET/CT staging. For this reason, in patients with a peripheral NSCLC more than 3 cm in larger diameter (but no more than 4 cm) our practice is to perform EBUS-TBNA only if risks factors for occult metastases, such as young age, visceral pleural invasion and higher standardized uptake value on PET are present (2,57,60). Otherwise we think that proceeding directly to surgery is a reasonable choice. For the same reason in this clinical scenario if a systematic EBUS-TBNA (including a sampling with almost three passes for each nodal station) is negative, a confirmatory videomediastinoscopy may be avoided.
Clinical stage IA (central T1abcN0); clinical stage IB (central T2aN0); Clinical stage II (T1abc-T2abN1; T2bN0, T3N0)
The prevalence of pathological N2 disease in clinical stage I NSCLC with negative mediastinum on CT and PET is considerably higher in central than peripheral tumours (21.6% versus 2.9%) (61). In clinical stage II NSCLC the false negative rate for CT and PET approaches to 25% (62-64). Given the high post-test probability of mediastinal neoplastic localization in these clinical scenarios, our practice is to perform EBUS-TBNA as first-line invasive staging technique. Given the relatively high NPV of EBUS-TBNA in these settings (5,6), a confirmatory videomediastinoscopy may be omitted.
Clinical stage IIIA (T1-2N2; T3N1; T4N0-1)
Since CT and PET have shown high false positive rates in mediastinal staging (2), enlarged mediastinal lymph nodes and/or PET positive mediastinal findings should be cytologically or histologically confirmed. In this clinical setting our practice is to prefer EBUS-TBNA over videomediastinoscopy as the first-line method to invasively stage the mediastinum. However, if the endoscopic procedure does not show malignant nodal involvement, a confirmatory videomediastinoscopy is always necessary.
Many reasons led us to the choice of an initial minimally invasive staging strategy using EBUS-TBNA over videomediastinoscopy in patients with potentially resectable NSCLC.
First, EBUS-TBNA and videomediastinoscopy have the same sensitivity and NPV but, compared with the surgical procedure, EBUS-TBNA is less invasive and associated with a lower morbidity. Moreover, overall costs for the endoscopic procedure are lower than those for videomediastinoscopy (2).
Second, we chose to use EBUS-TBNA because in the last 10 years we have considerably improved our experience with this technique, performing more than 70 procedures per year and consequently increasing our diagnostic yield. EBUS-TBNA, in fact, is operator-dependent, and its diagnostic yield is also related to the operator’s experience. Over time we have also increased our ability to sample small sized mediastinal lymph nodes and now we are able to use EBUS-TBNA with high diagnostic yield also in patients in whom the mediastinum appears normal, a condition defined by lymph nodes with short-axis diameters <1 cm on contrast-enhanced CT.
Third, although EUS-FNA is considered an accurate method for invasive staging of NSCLC (5,6,38), we prefer EBUS-TBNA because we believe it has some advantages. EBUS-TBNA is more likely to be able to make a difference in managing NSCLC patients suitable for operation than EUS-FNA. EUS-FNA has excellent access to stations 9, 8 and 7; however right and left paratracheal lymph nodes (stations 2 and 4) are difficult to biopsy with this technique. These nodes are those more frequently involved in NSCLC. The prevalence of mediastinal nodal metastases in stations 8 and 9, in fact, is very low ranging between 0.19% and 1.2% (8). Moreover, the presence of metastatic paratracheal lymph nodes has a big effect on patient management. In fact, in our practice all patients with nodal metastases in stations 2 and 4 are considered for neoadjuvant chemotherapy. On the contrary, many patients with metastases confined to stations 9 and 8 may be considered for primary operation, followed by adjuvant chemotherapy, without the need to preoperatively obtain a tissue confirmation of neoplastic nodal involvement (12).
A further consideration on the rationale of the use EBUS-TBNA over videomediastinoscopy in presurgical mediastinal staging of NSCLC is that some thoracic surgeons surgically treated only stage IIIA NSCLC patients with histologically confirmed mediastinal downstaging after neoadjuvant therapy, reserving definitive chemoradiation therapy to those without mediastinal downstaging. It is evident that the use of EBUS-TBNA as first-line diagnostic procedure in this clinical scenario avoids the risk performing a technically demanding re-mediastinoscopy.
Conclusions
EBUS-TBNA is now recommended by the published guidelines (5,6,38) as the technique of choice for the preoperative invasive mediastinal staging of NSCLC. When performed by a fully trained thoracic surgeon or interventional pulmonologist in an adequate environment, the sensitivity of EBUS-TBNA is higher than that of videomediastinoscopy. Therefore, this minimally invasive technique should be systematically offered to NSCLC patients, when preoperative mediastinal staging is required. However, we know that differences exist between thoracic surgeons regarding to the aggressiveness of preoperative mediastinal staging of NSCLC. As recently pointed out by Rocco et al. (65), in an analysis of the Society of Thoracic Surgeons (STS) and the ESTS combined database, including more than 70.000 lung resection, despite similarities in the respective guidelines, only 8% of patients were found to have N2 disease after lobectomy in the STS database compared with 14% in the European registry. It is likely that these differences in occult N2 disease are mainly due to the increasing tendency in Europe to proceed directly to surgery without neoadjuvant chemotherapy in patients with limited (single station, non bulky) N2 nodal involvement (65). With this strategy (upfront surgery followed by adjuvant chemotherapy for NSCLC N2 disease) the preoperative mediastinal assessment of the patient may stop at PET/CT, omitting an invasive mediastinal staging (66). Our current practice, however, is to use neoadjuvant chemotherapy (or chemoradiation therapy) in all patients with a neoplastic involvement of stations 2, 4 or 7 and to complete the multimodal treatment with surgery only if a downstaging may be documented. Following these strict indications, the preoperative assessment of these nodal stations is of paramount importance in all patients with clinical stage III NSCLC, to identify that subset of patients with a false-positive PET result who can be spared from induction therapy.
On the other hand, because most data from the literature show relatively high rates of occult N2 disease in some patients with a normal mediastinum on CT and/or PET and specific risk factors (some clinical stages I and clinical stage II NSCLC), it seems to us have a rationale using an invasive mediastinal staging also in these subsets of patients.
For these reasons, EBUS-TBNA with a systematic sampling has a central role in our current practice of thoracic surgeons regarding to the pre-treatment staging of NSCLC.
In the last years some studies have demonstrated the relatively favourable outcome of patients with surgically resected occult N2 NSCLC (58,67,68). If these initial results will be confirmed in larger studies, it is likely that in the future we can use a less aggressive preoperative mediastinal staging. Up to that time we will continue to submit our patients to aggressive preoperative mediastinal staging algorithms with EBUS-TBNA as first-line diagnostic technique.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Angelo Carretta) for the series “Minimally Invasive (Endoscopic) Mediastinal Staging of Lung Cancer” published in Mediastinum. The article has undergone external peer review.
Peer Review File: Available at http://dx.doi.org/10.21037/med-20-23
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-23). The series “Minimally Invasive (Endoscopic) Mediastinal Staging of Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical statement: the authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pandey D, Ramanathan P, Pandey R, et al. Mediastinal staging for non-small cell lung cancer revisited. It is being done under aegis of ICON and lung cancer consortium Asia. Indian J Cancer 2017;54:68-72. [Crossref] [PubMed]
- Carlens E. Mediastinoscopy: a method for inspection and tissue biopsy in the superior mediastinum. Dis Chest 1959;36:343. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- D’Andrilli A, Maurizi G, Venuta F, et al. Mediastinal staging: when and how? Gen Thorac Cardiovasc Surg 2020;68:725-32. [Crossref] [PubMed]
- Czarnecka-Kujawa K, Yasufuku K. The role of endobronchial ultrasound versus mediastinoscopy for non-small cell lung cancer. J Thorac Dis 2017;9:S83-97. [Crossref] [PubMed]
- Call S, Obiols C, Rami-porta R, et al. Video-assisted mediastinoscopic lymphadenectomy for staging non-small cell lung cancer. Ann Thorac Surg 2016;101:1326-33. [Crossref] [PubMed]
- Kuzdzał J, Zieliński M, Papla B, et al. Transcervical extended mediastinal lymphadenectomy: the new operative technique and early results in lung cancer staging. Eur J Cardiothorac Surg 2005;27:384-90. [Crossref] [PubMed]
- Kużdżał J, Szlubowski A, Grochowski Z, et al. Current evidence on transcervical mediastinal lymph nodes dissection. Eur J Cardiothorac Surg 2011;40:1470-3. [PubMed]
- Kuzdzał J, Zieliński M, Papla B, et al. The transcervical extended mediastinal lymphadenectomy versus cervical mediastinoscopy in non-small lung cancer staging. Eur J Cardiothorac Surg 2007;31:88-94. [Crossref] [PubMed]
- Melloni G, Casiraghi M, Bandiera A, et al. Transbronchial needle aspiration in lung cancer patients suitable for operation with positive mediastinal positron emission tomography. Ann Thorac Surg 2009;87:373-8. [Crossref] [PubMed]
- Melloni G, Bandiera A, Muriana P, et al. Combined use of TBNA and EBUS-TBNA in the preoperative staging of lung cancer patients. J Bronchology Interv Pulmonol 2011;18:311-6. [Crossref] [PubMed]
- Lin J, Fernandez F. Indications for invasive mediastinal staging for non-small cell lung cancer. J Thorac Cardiovasc Surg 2018;156:2319-24. [Crossref] [PubMed]
- Um SW, Kim HK, Jung SH, et al. Endobronchial ultrasound versus mediastinoscopy for mediastinal nodal staging of non-small-cell lung cancer. J Thorac Oncol 2015;10:331-7. [Crossref] [PubMed]
- Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guide transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 2011;142:1393-400.e1. [Crossref] [PubMed]
- Asano F, Aoe M, Ohsaki Y, et al. Complications associated with endobronchial ultrasound-guided transbronchial needle aspiration: a nationwide survey by the Japan Society for respiratory Endoscopy. Respir Res 2013;14:50. [Crossref] [PubMed]
- von Bartheld MB, Annema JT. Endosonography-related mortality and morbidity for pulmonary indications: a nationwide survey in the Netherlands. Gastrointest Endosc 2015;82:1009-15. [Crossref] [PubMed]
- Yasufuku K, Chiyo M, Sekine Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest 2004;126:122-8. [Crossref] [PubMed]
- Herth FJ, Eberhardt R, Vilmann P, et al. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax 2006;61:795-8. [Crossref] [PubMed]
- Adams K, Shah PL, Edmonds L, et al. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: systematic review and meta-analysis. Thorax 2009;64:757-62. [Crossref] [PubMed]
- Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer 2009;45:1389-96. [Crossref] [PubMed]
- Dong X, Qiu X, Liu Q, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the mediastinal staging of non-small cell lung cancer: a meta-analysis. Ann Thorac Surg 2013;96:1502-7. [Crossref] [PubMed]
- Sehgal IS, Dhooria S, Aggarwal AN, et al. Endosonography versus mediastinoscopy in mediastinal staging of lung cancer: systematic review and meta-analysis. Ann Thorac Surg 2016;102:1747-55. [Crossref] [PubMed]
- Ernst A, Anantham D, Eberhardt R, et al. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol 2008;3:577-82. [Crossref] [PubMed]
- Ceron L, Michieletto L, Zamperlin A. Mediastinal staging in lung cancer: a rational approach. Monaldi Arch Chest Dis 2009;71:170-5. [PubMed]
- Szlubowski A, Zielinsksi M, Soja J, et al. A combined approach of endobronchial and endoscopic ultrasound-guided transbronchial needle aspiration in the radiologically normal mediastinum in non-small cell lung cancer staging: a prospective trial. Eur J Cardiothorac Surg 2010;37:1175-9. [Crossref] [PubMed]
- Dooms C, Tournoy KG, Schuurbiers O, et al. Endosonography for mediastinal nodal staging of clinical N1 non-small cell lung cancer. Chest 2015;147:209-15. [Crossref] [PubMed]
- Rintoul RC, Tournoy KG, El Daly H, et al. EBUS-TBNA for the clarification of PET positive intra-thoracic lymph nodes: an international multi-centre experience. J Thorac Oncol 2009;4:44-8. [Crossref] [PubMed]
- Steinfort DP, Hew MJ, Irving LB. Bronchoscopic evaluation of the mediastinum using endobronchial ultrasound: a description of the first 216 cases carried out at an Australian tertiary hospital. Intern Med J 2011;41:815-24. [Crossref] [PubMed]
- Detterbeck F, Puchalski J, Rubinowitz A, et al. Classification of the thoroughness of mediastinal staging of lung cancer. Chest 2010;137:436-42. [Crossref] [PubMed]
- Jeebun V, Harrison RN. Understanding local performance data for EBUS-TBNA: insights from an unselected case series at a high volume UK center. J Thorac Dis 2017;9:S350-62. [Crossref] [PubMed]
- Lee HS, Lee GK, Lee HS, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging non-small cell lung cancer: how many aspirations per target lymph node station? Chest 2008;134:368-74. [Crossref] [PubMed]
- Diacon AH, Schuurmans MM, Theron J, et al. Transbronchial needle aspirates: how many passes per target site? Eur Respir J 2007;29:112-6. [Crossref] [PubMed]
- Herth FJ, Eberhardt R, Krasnik M, et al. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically and PET normal mediastinum in patients with lung cancer. Chest 2008;133:887-91. [Crossref] [PubMed]
- Nakajima T, Yasufuku K, Saegusa F, et al. Rapid on-site cytologic evaluation during endobronchial ultrasound-guided transbronchial needle aspiration of nodal staging in patients with lung cancer. Ann Thorac Surg 2013;95:1695-9. [Crossref] [PubMed]
- Sehgal IS, Dhooria S, Aggarwal AN, et al. Training and proficiency in endobronchial ultrasound-guided transbronchial needle aspiration. A systematic review. Respirology 2017;22:1547-57. [Crossref] [PubMed]
- Naur TMH, Konge L, Nayahangan LJ, et al. Training and certification in endobronchial ultrasound-guided transbronchial needle aspiration. J Thorac Dis 2017;9:2118-23. [Crossref] [PubMed]
- Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Endoscopy 2015;47:545-59. [PubMed]
- Micames CG, Mccrory DC, Pavey DA, et al. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging. A systematic review and metaanalysis. Chest 2007;131:539-48. [Crossref] [PubMed]
- Zhang R, Ying K, Shi L, et al. Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal lymph node staging of lung cancer staging: a meta-analysis. Eur J Cancer 2013;49:1860-7. [Crossref] [PubMed]
- Korevaar DA, Crombag LM, Cohen JF, et al. Added value of combined endobronchial and oesophageal endosonography for mediastinal nodal staging in lung cancer. A systematic review and meta-analysis. Lancet Respir Med 2016;4:960-8. [Crossref] [PubMed]
- Dhooria S, Aggarwal AN, Gupta D, et al. Utility and safety of endoscopic ultrasound with bronchoscope-guided fine-needle aspiration in mediastinal lymph node sampling: staging: a systematic review and meta-analysis. Respir Care 2015;60:1040-50. [Crossref] [PubMed]
- Decaluwé H, Dooms C, D’Journo XB, et al. Mediastinal staging by videomediastinoscopy in clinical N1 non-small cell lung cancer: a prospective multicentre study. Eur Respir J 2017;50:1701493. [Crossref] [PubMed]
- Thornblade LW, Wood DE, Mulligan MS, et al. Variability in invasive mediastinal staging for lung cancer: a multi-center regional study. J Thorac Cardiovasc Surg 2018;155:2658-71.e1. [Crossref] [PubMed]
- Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 2005;80:2051-6. [Crossref] [PubMed]
- Ost DE, Niu J, Elting LS, et al. Quality gaps and comparative effectiveness in lung cancer staging and diagnosis. Chest 2014;145:331-5. [Crossref] [PubMed]
- Bendzsak A, Waddell TK, Yasufuku K, et al. Invasive mediastinal staging. Guideline concordance. Ann Thorac Surg 2017;103:1736-41. [Crossref] [PubMed]
- Bousema JE, Heineman DJ, Dijkgraaf MGW, et al. Adherence to the mediastinal staging guideline and unforeseen N2 disease in patients with resectable non-small cell lung cancer; nationwide results from the Dutch lung cancer audit – surgery. Lung Cancer 2020;142:51-8. [Crossref] [PubMed]
- De Leyn P, Stroobants S, De Wever W, et al. Prospective comparative study of integrated positron emission tomography-computed tomography scan compared with remediastinoscopy in the assessment of residual mediastinal lymph node disease after induction chemotherapy for mediastinoscopy-proven stage IIIA-N2 non-small cell lung cancer: a Leuven lung cancer group study. J Clin Oncol 2006;24:3333-9. [Crossref] [PubMed]
- Mateu-Navarro M, Rami-Porta R, Bastus-Piulats R, et al. Remediastinoscopy after induction chemotherapy in non-small cell lung cancer. Ann Thorac Surg 2000;70:391-5. [Crossref] [PubMed]
- de Cabanyes Candela S, Detterbeck FC. A systematic review of restaging after induction therapy for stage IIIa lung cancer. Prediction of pathologic stage. J Thorac Oncol 2010;5:389-98. [Crossref] [PubMed]
- Herth FJ, Annema JT, Eberhardt R, et al. Endobronchial ultrasound with transbronchial needle aspiration for restaging the mediastinum in lung cancer. J Clin Oncol 2008;26:3346-50. [Crossref] [PubMed]
- Stamatis G, Fechner S, Hillejan L, et al. Repeat mediastinoscopy as a restaging procedure. Pneumologie 2005;59:862-6. [Crossref] [PubMed]
- Wang J, Welch K, Wang L, et al. Negative predicted value of positron emission tomography and computed tomography for stage T1-2N0 non-small cell lung cancer: a meta-analysis. Clin Lung Cancer 2012;13:81-9. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Eloubeidi MA. Routine mediastinoscopy and esophageal ultrasound fine-needle aspiration in patients with non-small cell lung cancer who are clinically N2 negative. Chest 2006;130:1791-5. [Crossref] [PubMed]
- Meyers BF, Haddad F, Siegel BA, et al. Cost-effectiveness of routine mediastinoscopy in computed tomography-and positron emission tomography-screened patients with stage I lung cancer. J Thorac Cardiovasc Surg 2006;131:822-9. [Crossref] [PubMed]
- Carnevale A, Milanese G, Sverzellati N, et al. A novel prediction model for the probability of mediastinal lymph node metastases detected by endobronchial ultrasound-transbronchial needle aspiration in non-small cell lung cancer: possible applications in clinical decision-making. Mediastinum 2018;2:43. [Crossref]
- Kim MP, Correa AM, Hofstetter WL, et al. Occult stage IIIA-N2 patient have excellent overall survival with initial surgery. J Thorac Dis 2018;10:6670-6. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposal for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- O’Connell OJ, Almeida FA, Simoff MJ, et al. A prediction model to help with the assessment of adenopathy in lung cancer. Am J Respir Crit Care Med 2017;195:1651-60. [Crossref] [PubMed]
- Lee PC, Port JL, Korst RJ, et al. Risk factors for occult mediastinal metastases in clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:177-81. [Crossref] [PubMed]
- Pozo-Rodríguez F, Martin de Nicola JL, Sanchez-nistal MA, et al. Accuracy of helical computed tomography and [18] fluorodeoxyglucose positron emission tomography for identifying lymph node mediastinal metastases in potentially resectable non-small-cell lung cancer. J Clin Oncol 2005;23:8348-56. [Crossref] [PubMed]
- Serra M, Cirera L, Rami-Porta R, et al. Routine positron emission tomography (PET) and selective mediastinoscopy is as good as routine mediastinoscopy to rule out N2 disease in non-small cell lung cancer (NSCLC). J Clin Oncol 2006;24:7031. [Crossref]
- Verhagen AF, Bootsma GP, Tjan-Heijnen VC, et al. FDG-PET in staging lung cancer: how does it change the algorithm? Lung Cancer 2004;44:175-81. [Crossref] [PubMed]
- Rocco G, Nason K, Brunelli A, et al. Management of stage IIIA (N2) non-small-cell lung cancer: a transatlantic perspective. Eur J Cardiothorac Surg 2016;49:1025-7. [Crossref] [PubMed]
- Lim E, McElnay PJ, Rocco G, et al. Invasive mediastinal staging is irrelevant for PET/CT positive N2 lung cancer if the primary tumour and ipsilateral lymph nodes are resectable. Lancet Respir Med 2015;3:e32-3. [Crossref] [PubMed]
- Defranchi SA, Cassivi SD, Nichols FC, et al. N2 disease in T1 non-small-cell lung cancer. Ann Thorac Surg 2009;88:924-8. [Crossref] [PubMed]
- Cho HJ, Kim SR, Kim HR, et al. Modern outcome and risk analysis of surgically resected occult N2 non-small cell lung cancer. Ann Thorac Surg 2014;97:1920-5. [Crossref] [PubMed]
Cite this article as: Melloni G, Mazza F, Venturino M, Turello D. Role of endobronchial ultrasound-guided transbronchial needle aspiration in staging of lung cancer: a thoracic surgeon’s perspective. Mediastinum 2021;5:2.