A narrative review of the role of endoscopic ultrasound (EUS) in lung cancer staging
Introduction
Background
Endoscopic ultrasound (EUS) is a technique that combines endoscopy and ultrasound. Born some 40 years ago, it’s now a well-established tool for the diagnosis and staging of gastrointestinal (GI) and pancreato-biliary diseases. Initially considered an imaging diagnostic technique, it subsequently developed as a diagnostic and therapeutic tool, with cytology and histological diagnosis and therapeutic procedures made possible by the technological advancement.
The EUS’ diagnostic capabilities have increased over time, with improved imaging systems (processors, echoendoscopes) and the development of enhanced imaging functions such as contrast harmonic EUS (CH-EUS) and EUS elastography.
Currently EUS plays an important role in the staging, diagnosis and treatment of pancreatic-biliary, esophageal, gastric and rectal diseases. In the mediastinum, the EUS has brought the important advantage of a minimal-invasive diagnostic approach for the diagnosis and staging of malignant and benign conditions; with the real-time possibility of sampling lesions or lymph nodes.
Objectives: endosonography role in lung cancer staging
Lung cancer is one of the most common solid malignant disease, with non-small-cell lung cancer (NSCLC) being its most common variant. Accurate staging is required for planning the most appropriate treatment (1,2). Surgery or local radiotherapy are the elective treatment for localized disease. However, the presence of mediastinal malignant lymph nodes can affect the role of surgery as a first-line therapy; and for diffused-NSCLC or a small cell lung cancer (SCLC), chemotherapy and/or radiotherapy are recommended instead (3).
Computed tomography (CT) and/or positron emission fluorodeoxyglucose-tomography (FDG-PET) are used for the assessment of primary lung lesion and metastatic disease, including mediastinal lymph nodes. Although the detection of enlarged lymph nodes at CT or FDG-PET scan is suggestive of a malignant involvement (4,5), the accuracy of radiological techniques in mediastinal staging is suboptimal: histological diagnosis is required to confirm metastatic disease (6-8), and assess the potential for local surgical resection.
Mediastinoscopy is a well-established technique for an accurate assessment of mediastinal lymph nodes tumor involvement; but it is a very invasive technique, with a high costs and morbidity (9). Therefore, a less-invasive technique is desirable to stage the tumors, and tailor the best treatment for each patient.
EUS with fine needle aspiration (FNA) and/or fine needle biopsy (FNB), and endobronchial ultrasound (EBUS) with transbronchial needle aspiration (TBNA), represent a minimally invasive and cheaper alternative to surgery for the diagnosis and staging of mediastinal pathology (10-14). These techniques are well-tolerated by the patient, safe and usually performed under general anesthesia (15,16).
In 2013–2014 endosonography was considered as the initial test of choice for the diagnosis and staging of lymph nodes tissue (17,18) in lung cancer patients, reducing the requirement for thoracotomies and costs (19,20). Moreover, the combined use of EUS-FNA and EBUS-TBNA has been demonstrated to further increase the diagnostic accuracy (DA) for mediastinal staging in lung cancer, when compared to the two techniques used individually (21). This approach was therefore included in the European Societies’ combined guidelines of Gastrointestinal Endoscopy (ESGE) and the European Society of Thoracic Surgeons (ESTS) and the European Respiratory Society (ERS) (22,23). A recent meta-analysis (24), confirmed that the combined use of EBUS and EUS significantly improves the sensitivity in detecting mediastinal nodal metastases, reducing the need for surgical staging; and without significant differences in sensitivity rates (Se) and negative predictive value (NPV) among those studies where either EBUS or EUS are performed as the initial staging.
The combined approach from the esophagus/stomach (EUS) and airways (EBUS) allows for the evaluation of equal and distinct types of mediastinal lymph nodes, enhancing the complementary role of these techniques (Figure 1).
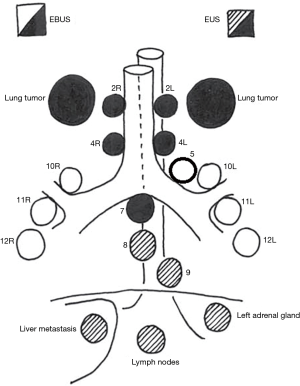
In lung cancer, there isn’t a single technique that would allow access to all the mediastinal lymph nodes for the sampling of tissue (22,23). Different studies confirmed the increase of the overall sensitivity of the combined use of EUS-FNA and EBUS-TBNA in the staging of lung cancer, when compared to the use of individual techniques. Sensitivity was demonstrated to increase from 45–92% respectively of the EUS or EBUS alone, to 68–100%. In addition, NPV increases from 67–93% to 91–100% when using the two techniques in combination (25-30). We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/med-20-51).
Methods
Which type of lymph nodes stations and abdominal structures can be evaluated by EUS?
Anatomical knowledge is fundamental in the understanding of the integrated role of EUS and EBUS. From the esophagus, EUS + FNA/FNB, can visualize the following mediastinal lymph nodes stations: stations 2L, 4L (high and lower left paratracheal nodes), station 7 (subcarinal nodes), stations 8 and 9 (lower mediastinal modes).
EUS + FNA/FNB also represents an important tool for the diagnosis of lung tumors close to the esophagus and infra diaphragmatic structures: such as retroperitoneal lymph nodes close to the aorta and the celiac trunk; and the abdominal lymph nodes stations (i.e., hepatic hylum, splenic, perigastric) or metastatic lesions in left liver lobe and left adrenal gland (13,22,23).
Stations 2R and 4R (right paratracheal) are difficult to reach with EUS-FNA from the esophagus, because the trachea interposes between the transducer and the lymph node, limiting the view of this area. They are accessed more easily by EBUS-TBNA; however, in case of large lymph nodes (>2 cm), the visualization and sampling could be possible (Figure 2A,B).
Paraortic stations 5 and 6 (predominantly affected by left upper lobe tumors) can be visualized by EUS; but they can rarely be sampled because of the interposition of the pulmonary artery and the aorta. Therefore, surgical staging by video-assisted thoracic surgery (VATS) is the method of choice.
Lung tumors and lymph nodes in stations 10, 11, and 12 (right/left) cannot be reached by EUS-FNA, but they can be easily sampled with the use of EBUS-TBNA.
Equipments
EUS-scopes
The EUS equipment consists of an ultrasound processor connected to an echoendoscope, with an ultrasound transducer (electronic in the newest generation) attached at the distal tip of the instrument. The endoscope in return is connected to a standard video processor, permitting the endoscopic visualization: the complete system allows for simultaneous endoscopic and ultrasound imaging.
Nowadays, several systems are available from different companies; and they are in constant evolution, to ensure the maximum level of image resolution, patients’ safety, and the comfort of the operator during the proceedings.
Echoendoscopes work at variable frequencies, the most common ranging between 5 and 12 MHz: higher frequencies allow a better resolution, but limit the penetration of the ultrasound beam.
There are two types of echoendoscopes, radial and linear (31). Radial transducers have individual piezoelectric elements of transducer around the distal tip in a 360° radial-array, producing an ultrasound image in a plane perpendicular to the long axis of the echoendoscope. They are used only for diagnostic purposes, since they do not allow for tissue sampling.
Linear EUS-scopes allow the real-time tissue sampling of targeted lesions, and represent the instrument of choice in for the staging and tissue sampling in lung cancer patients. They are therefore relevant to this review; unlike radial scopes who have an exclusive diagnostic role.
There are three major manufacturers for the production of linear echoendoscopes: Fujifilm Endoscopy (Fujifilm Europe GmbH, Germany), Olympus (Olympus Europa SE and Co. KG, Hamburg, Germany) and Pentax (Pentax Europe GmbH, Hamburg, Germany). The different characteristics of the three brands are listed in Table 1.
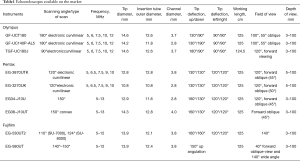
Full table
All currently available electronic linear instruments produce ultrasound images in a parallel plane to the long axis of the echoendoscope, usually with a sector width between 100° and 180°. This ultrasound image orientation is a key point for the sampling of tissue: the EUS devices are advanced from the distal tip of the echoendoscope in the same plane as the ultrasound image, allowing for the simultaneous visualization of the target lesion and the EUS device. In addition, all linear instruments incorporate an elevator at the distal end of the echoendoscope working channel, allowing for the complete directional control of the biopsy needle.
Ultrasound processors
Each echoendoscopic brand works with specific ultrasound echo-processors. The three major manufacturers, Fujifilm Endoscopy (Fujifilm Europe GmbH, Germany), Olympus (Olympus Europa SE and Co. KG, Hamburg, Germany) and Hitachi (Hitachi Medical Systems Europe, Zug, Switzerland), produce different types of ultrasound processor, compatible their echoendoscopes (31).
Table 2 reports the last-generation available ultrasound processors related to three different echoendoscopes companies.
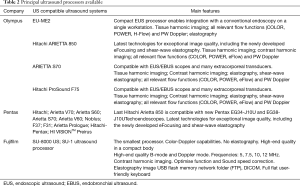
Full table
US-applied additional tools
New processors incorporate additional imaging software, including Power Doppler, tissue elastography and contrast-enhanced EUS (CE-EUS). All these tools complete the diagnostic phase of staging, and help the endosonographer in guiding the biopsy’s needle.
The differentiation between malignant and benign lymph nodes by standard cross-sectional imaging techniques (abdominal ultrasound, CT scan, magnetic resonance and EUS) is based mainly on morphological aspects, size and topographic distribution. However, in lung cancer the use of size alone accounts for the low DA, because up to 30% of lymph-nodes <5 mm are malignant (Figure 3) (32).
Elastography
Ultrasound elastography measures the tissue’s stiffness, by evaluating the changes in the EUS image before and after the application of a determined pressure with the ultrasound probe. This is based on the hypothesis that normal tissues are soft, and deform more than malignant which are stiffer. Elastography has been applied in the study of different tumors and plays a key role in lymph nodes characterization, by helping to differentiate benign versus malignant tissues, and guiding biopsy (33).
Elastography can measure the degree of tissue elasticity by means of two modalities: qualitative or semi-quantitative.
Qualitative elastography differentiates lesions according to their elasticity score on a color map, on a sliding scale from red-yellow (soft) to blue (stiff); this measurement is liable to subjective evaluation, and therefore operator-dependent.
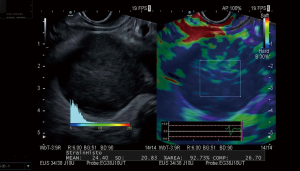
Semi-quantitative technique is more objective, with the measurement of parameters called “strain ratio (SR)” and “strain histogram (SH)”. The first is obtained by placing a “region of interest” (ROI) box inside the target lesion/lymph node (ROI A), and a second ROI box (ROI B) in a neighboring region representing the “normal tissue” (ROI B). The SR value is the percentage B/A. The Strain-histogram permits the graphical representation (histogram) of color distribution (pixels) in a ROI. The X-axis in the histogram represents the elasticity of the tissue/lymph node, from 0 (softest) to 255 (hardest). The Y-axis represents the number of pixels in each elasticity level in the ROI. The mean value of the histogram corresponds to the global hardness or elasticity of the studied region (Figure 4). This modality is considered the most objective method to measure tissue stiffness.
In lung cancer, elastography has demonstrated to show good sensitivity (89.4%) and a high NPV (95.1%) in the staging of mediastinal lymph-nodes, especially associated to ultrasound size of suspect lymph node (>1 cm) and it may facilitate the selection of the most suspicious lymph nodes to biopsy (34,35), or a more suspicious zone inside a lymph node. The DA of the elastography is significantly influenced by the operator’s experience, and the location of the lesion: the accuracy of the evaluation of areas in proximity with bones or big vessels, is tampered by the flow interference with waves transmission.
Color Doppler ultrasound and CH-EUS
Color Doppler ultrasound (color Doppler imaging) has been used to better characterize the lymph nodes and the ratio FNA/FNB. It improves the differentiation of lymph nodes by displaying the macro-vessels architecture. Inflammatory lymph nodes are typically more vascularized, without changes of the predominant hilar vessel architecture. Metastatic lymph nodes on the contrary, present peripheral or mixed vascularity and lose the hilar type vascularization (32). Color Doppler ultrasound can also guide the needle during biopsy to avoid the bigger vascular structures, and prevent bleeding.
CH-EUS imaging is another adjunctive tool for the detection of malignant lymph nodes. It works with intravenous administration of a new II generation contrast, followed by 10–20 mL of saline which generates micro-bubbles (sulphur hexafluoride) in the vessels with a diameter of approximately 0.1–0.4 mm. CH-EUS combines a method of a very high-resolution, with the contrast-enhancing of the lymph nodes’ capillary bed. Working on the assumption that that the capillary bed of a metastatic tissue is destroyed, the contrast should be less visible.
Different studies reported both Doppler imaging (CE-EUS) and CH-EUS results, with mixed aftermath [Se: 60–100%, specificity (Sp): 85–100%] (36).
In a recent meta-analysis (37), CH-EUS showed an accuracy (pooled Se: 87.7% and Sp: 91.8%), comparable to elastography and EUS-FNA; this suggests a role for CH-EUS (eventually combined with elastography) in the staging of lymph-node in lung cancer.
Needles and additional tools
EUS needles (FNA/FNB)
Different sampling needles are available on the market, with differ in morphology of the tip, stylet material and flexibility. The choice is based on local factors like the ultrasonographer’s preference, or the availability of rapid on-site evaluation (ROSE) of the sample. Two types of needles types are available, for cytology and histology respectively (FNA and FNB). FNB needles are also important for molecular studies. The needles available on the market and their characteristics are listed in Table 3.
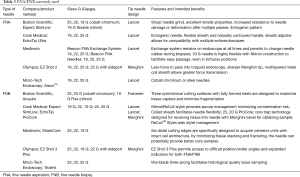
Full table
Different needles size are used in EUS to characterize lymph nodes: 19, 20, 22, 25 Gauge (G). ESGE Guidelines (38) recommend the use of 25 or 22 G needles for routine EUS-guided sampling of solid masses or lymph nodes, with FNA or FNB equally recommended. However, when the primary aim of sampling is to obtain a core of tissue (FNB), ESGE suggests the using of 19 G FNA or FNB needles or 22 G FNB needles.
Biopsy techniques
For lymph nodes biopsy, ESGE recommends to use the 10 mL syringe suction during EUS-guided sampling with 25 or 22 G needles or other types of needles, suggesting the neutralizing of negative pressure in the needle before withdrawing from the targeted lesion.
The “stylet slow pull” technique consists on the slow removal of the needle stylet during biopsy, creating a minimal negative pressure within the needle, which could enhance the sampling. Retrospective studies suggest advantages of this technique over standard suction, but few randomized controlled studies (RCTs) comparing DA between the two methods are available. In a recent meta-analysis (39) of seven RCTs the suction method was not better than the over slow-pull technique; the latter however can reduce the rate of blood contamination.
The “fanning technique” (38) consists on moving the needle with the scope elevator during the lymph node sampling, in order to sample different part of the lymph nodes.
On-site cytological evaluation
ROSE of cytological samples obtained by FNA, is the immediate evaluation of the material obtained by the EUS-guided biopsy by a pathologist or cyto-technician in the endoscopic room, in order to assess the presence of “diagnostic material”. ESGE Guidelines equally recommends sampling with or without ROSE. ROSE is not available in many centers, due to the high costs and the required expertise. When available, ROSE represents an advantage because it reduces the examination time and sampling, and the need for further repeated EUS because of a non-diagnostic sample.
When ROSE is not available, ESGE suggests multiple sampling: three or four needle passes with FNA, or two or three passes using FNB needles respectively (38).
How to do it: lymph nodes staging and sampling
Endoscopic room organization
A specific endoscopic room layout is fundamental for a good EUS execution, with the endosonographer near to the patient and ultrasound or endoscopic processors. The correct position of the patients on left lateral decubitus. The ultrasound processor screen must be well-visible and accessible by the operator; both the ultrasound and endoscopic screens should be well visible by the medical staff during the diagnostic and sampling phase.
As for the anesthesia, EUS could be performed in moderate sedation without the anesthesiologist’s assistance, or in deep sedation. The latter has the advantage of a more-quiet diagnostic evaluation, and an easier biopsy of the target lesions.
A readily accessible “biopsy location” must be prepared with all the required materials (formalin, slides, colorants, etc.). If ROSE is available, a microscope must also be available direct evaluation of samples.
How to do the staging (mediastinal evaluation and abdominal stations)
To optimize the EUS-evaluation, a standardized and methodical way of working is required. The operator position and keeping the linear EUS scope straight is fundamental, to permit the transmission of each small endoscopist’s movements to the tip of the scope, and leave the right hand free to use the EUS processor or devices.
Usually for a complete mediastinal evaluation, the instrument is positioned in the stomach at the level of the body; and the evaluation is performed by gently and slowly withdrawing the scope with a 360° rotation. The patient is positioned in left lateral decubitus with anestesiologist’s assistance and deep sedation when required.
By rotating clockwise the scope along side the lesser gastric curvature and going right to left i.e., from the left lobe of the liver lobe towards the spleen, it is possible to evaluate the following abdominal stations: the left liver lobe (II, III, IV and I segments), hepatic hilum, pancreatic body and tail, splenic hilum, spleen, left adrenal gland (Figure 4), left kidney, perigastric, perisplenic, along splenic and mesenteric vessels and the celiac trunk lymph nodes stations.
It is also possible to complete the abdominal staging by reaching the second part of the duodenum with the endoscope, and rotating anti-clockwise from the superior mesenteric vessels to the posterior big vessels (aorta, cava vein). This will allow to evaluate the superior mesenteric vessels lymph nodes, the pancreatic head, the uncinate process and neck, the hepatic hylum, parts of the right lobe of the liver, the gallbladder, the peri-duodenal space, and the paraortic and paracaval lymph nodes.
Withdrawing the scope from the stomach towards the esophagus with a 360°-rotation of the linear echoendoscope (for example from the thoracic aorta to the aorta), it is possible to evaluate by EUS the cardiac region and diaphragm; and also to evaluate and biopsy the following mediastinal stations or neighboring parenchymal lung lesions: the high and lower left paratracheal nodes (2L, 4L stations), the subcarinal nodes (station 7), and the lower mediastinum lymph nodes (8–9 stations).
As already mentioned, the right paratracheal lymph nodes (stations 2R and 4R) can be visualized and sampled if their size is >2 cm. Stations 5 and 6 can also be visualized by EUS, but can they can rarely be sampled without crossing the pulmonary artery and aorta (in this case VATS is the method of choice).
EUS can also visualize the lymph nodes of the aorto-pulmonary window along the left subclavian artery, and higher lymph nodes of the neck (carotid artery-and jugular vein) as well as the thyroid parenchyma.
How to characterize a pathologic lymph node with EUS
Using the all above mentioned tools it is possible to characterize a malignant lymph node, which appears at B-mode evaluation with size >1 cm (although it is common to encounter smaller lymph nodes with a typical pathological aspect), homogeneously hypoechoic, and with rounded shapes. It is also possible to see necrotic parts appearing as cystic gaps in the bigger pathologic lymph nodes, not vascularized at CH-EUS.
After the basic B-mode evaluation, the elastography evaluation represents an additional tool to characterize malignant nodes. A pathologic lymph node appears at elastography as rigid (homogeneously blue at qualitative evaluation), with low a strain ration, and a totally left distribution color-spike at histograms in semi-quantitative evaluation.
The other possibility is to evaluate the lymph nodes with Doppler or CH-EUS, to avoid the necrotic tissue inside the malignant nodes and optimize the diagnostic sampling.
B-mode aspect, elastography, Doppler and CH-EUS are additional tools to be used jointly to increase the DA of EUS-guide biopsy.
Summary
EUS represents an important and complementary examination for the staging of lung cancer, which completes the EBUS staging. It also offers the advantage of evaluating both mediastinal and abdominal lymph nodes stations and structures. With the support of the latest equipment, EUS represents an essential and mini-invasive method for the staging of lung cancer.
Acknowledgments
Thanks to Paolo M. Rizzi, MD, for contribution in editing and English writing.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Angelo Carretta) for the series “Minimally Invasive (Endoscopic) Mediastinal Staging of Lung Cancer” published in Mediastinum. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/med-20-51
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-51). The series “Minimally Invasive (Endoscopic) Mediastinal Staging of Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374-403. [Crossref] [PubMed]
- Rivera MP, Mehta AC; American College of Chest Physicians. Initial diagnosis of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:131S-48S.
- Vansteenkiste J, De Ruysscher D, Eberhardt WE, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi89-98. [Crossref] [PubMed]
- Libshitz HI, McKenna RJ Jr. Mediastinal lymph node size in lung cancer. AJR Am J Roentgenol 1984;143:715-8. [Crossref] [PubMed]
- Lardinois D, Weder W, Hany TF, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med 2003;348:2500-7. [Crossref] [PubMed]
- Tournoy KG, Maddens S, Gosselin R, et al. Integrated FDG-PET/CT does not make invasive staging of the intrathoracic lymph nodes in non-small cell lung cancer redundant: a prospective study. Thorax 2007;62:696-701. [Crossref] [PubMed]
- De Wever W, Stroobants S, Coolen J, et al. Integrated PET/CT in the staging of nonsmall cell lung cancer: technical aspects and clinical integration. Eur Respir J 2009;33:201-12. [Crossref] [PubMed]
- Fischer BM, Mortensen J, Hansen H, et al. Multimodality approach to mediastinal staging in non-small cell lung cancer. Faults and benefits of PET-CT: a randomised trial. Thorax 2011;66:294-300. [Crossref] [PubMed]
- Detterbeck FC, Jantz MA, Wallace M, et al. Invasive mediastinal staging of lung cancer: ACCP evidence-based clinical practice guidelines (2ndedition). Chest 2007;132:202S-20S. [Crossref] [PubMed]
- Pedersen BH, Vilmann P, Folke K, et al. Endoscopic ultrasonography and real-time guided fine-needle aspiration biopsy of solid lesions of the mediastinum suspected of malignancy. Chest 1996;110:539-44. [Crossref] [PubMed]
- Verma A, Jeon K, Koh WJ, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of central lung parenchymal lesions. Yonsei Med J 2013;54:672-8. [Crossref] [PubMed]
- Silvestri GA, Hoffman BJ, Bhutani MS, et al. Endoscopic ultrasound with fine-needle aspiration in the diagnosis and staging of lung cancer. Ann Thorac Surg 1996;61:1441-5; discussion 1445-6. [Crossref] [PubMed]
- Vilmann P, Annema J, Clementsen P. Endosonography in bronchopulmonary disease. Best Pract Res Clin Gastroenterol 2009;23:711-28. [Crossref] [PubMed]
- Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA 2010;304:2245-52. [Crossref] [PubMed]
- Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology 1997;112:1087-95. [Crossref] [PubMed]
- Herth FJ, Eberhardt R, Vilmann P, et al. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax 2006;61:795-8. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Rintoul RC, Glover MJ, Jackson C, et al. Cost effectiveness of endosonography versus surgical staging in potentially resectable lung cancer: a health economics analysis of the ASTER trial from a European perspective. Thorax 2014;69:679-81. [Crossref] [PubMed]
- Sharples LD, Jackson C, Wheaton E, et al. Clinical effectiveness and costeffectiveness of endobronchial and endoscopic ultrasound relative to surgical staging in potentially resectable lung cancer: results from the ASTER randomised controlled trial. Health Technol Assess 2012;16:1-75. iii-iv. [Crossref] [PubMed]
- Zhang R, Ying K, Shi L, et al. Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal lymph node staging of lung cancer: a meta-analysis. Eur J Cancer 2013;49:1860-7. [Crossref] [PubMed]
- Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Eur Respir J 2015;46:40-60. [Crossref] [PubMed]
- Vilmann P, Clementsen PF, Colella S, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Endoscopy 2015;47:545-59. [Crossref] [PubMed]
- Korevaar DA, Crombag LM, Cohen JF, et al. Added value of combined endobronchial and oesophageal endosonography for mediastinal nodal staging in lung cancer: a systematic review and meta-analysis. Lancet Respir Med 2016;4:960-8. [Crossref] [PubMed]
- Vilmann P, Krasnik M, Larsen SS, et al. Transesophageal endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) biopsy: a combined approach in the evaluation of mediastinal lesions. Endoscopy 2005;37:833-9. [Crossref] [PubMed]
- Wallace MB, Pascual JM, Raimondo M, et al. Minimally invasive endoscopic staging of suspected lung cancer. JAMA 2008;299:540-6. [Crossref] [PubMed]
- Herth FJ, Krasnik M, Kahn N, et al. Combined endoscopic-endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes through a single bronchoscope in 150 patients with suspected lung cancer. Chest 2010;138:790-4. [Crossref] [PubMed]
- Szlubowski A, Zieliński M, Soja J, et al. A combined approach of endobronchial and endoscopic ultrasound-guided needle aspiration in the radiologically normal mediastinum in non-small-cell lung cancer staging--a prospective trial. Eur J Cardiothorac Surg 2010;37:1175-9. [Crossref] [PubMed]
- Liberman M, Sampalis J, Duranceau A, et al. Endosonographic mediastinal lymph node staging of lung cancer. Chest 2014;146:389-97. [Crossref] [PubMed]
- Oki M, Saka H, Ando M, et al. Endoscopic ultrasound-guided fine needle aspiration and endobronchial ultrasound-guided transbronchial needle aspiration: Are two better than one in mediastinal staging of non-small cell lung cancer? J Thorac Cardiovasc Surg 2014;148:1169-77. [Crossref] [PubMed]
- ASGE Technology Committee, Murad FM, Komanduri S, et al. Echoendoscopes. Gastrointest Endosc 2015;82:189-202. [Crossref] [PubMed]
- Hocke M, Ignee A, Dietrich D. Role of contrast-enhanced endoscopic ultrasound in lymph nodes. Endosc Ultrasound 2017;6:4-11. [Crossref] [PubMed]
- Iglesias-Garcia J, Lindkvist B, Lariño-Noia J, et al. Endoscopic ultrasound elastography. Endosc Ultrasound 2012;1:8-16. [Crossref] [PubMed]
- Fujiwara T, Nakajima T, Inage T, et al. The combination of endobronchial elastography and sonographic findings during endobronchial ultrasound-guided transbronchial needle aspiration for predicting nodal metastasis. Thorac Cancer 2019;10:2000-5. [Crossref] [PubMed]
- Dietrich CF, Jenssen C, Herth FJ. Endobronchial ultrasound elastography. Endosc Ultrasound 2016;5:233-8. [Crossref] [PubMed]
- Fusaroli P, Napoleon B, Gincul R, et al. The clinical impact of ultrasound contrast agents in EUS: a systematic review according to the levels of evidence. Gastrointest Endosc 2016;84:587-96.e10. [Crossref] [PubMed]
- Lisotti A, Ricci C, Serrani M, et al. Contrast-enhanced endoscopic ultrasound for the differential diagnosis between benign and malignant lymph nodes: a meta-analysis. Endosc Int Open 2019;7:E504-13. [Crossref] [PubMed]
- Polkowski M, Jenssen C, Kaye P, et al. Technical Aspects of Endoscopic Ultrasound (EUS)-guided Sampling in Gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline - March 2017. Endoscopy 2017;49:989-1006. [Crossref] [PubMed]
- Capurso G, Archibugi L, Petrone MC, et al. Slow-pull compared to suction technique for EUS-guided sampling of pancreatic solid lesions: a meta-analysis of randomized controlled trials. Endosc Int Open 2020;8:E636-43. [Crossref] [PubMed]
Cite this article as: Rossi G, Petrone MC, Arcidiacono PG. A narrative review of the role of endoscopic ultrasound (EUS) in lung cancer staging. Mediastinum 2021;5:1.