
The role of induction therapy for thymic malignancies: a narrative review
Introduction
Thymic malignancies are relatively rare epithelial neoplasms, with an incidence of approximately 2.5 to 3.2 per million people (1,2). Surgical resection is considered the mainstay of curative treatment (3-5), however, approximately 30% of thymic epithelial tumors (TET) are identified as locally advanced and carry a significant risk of incomplete resection and worse outcomes (6,7). The prognostic importance of obtaining an R0 (complete) resection and the known sensitivity of TETs to chemotherapy and radiotherapy has naturally led to the utilization of induction therapy in an attempt to improve complete resection rates, particularly for marginally resectable disease (8). The potential advantages of induction therapy include tumor down-staging, increased likelihood of an R0 resection, and prevention of systemic progression (9). Since the introduction of induction therapy for thymic malignancies in the 1980s (10), several retrospective studies and phase II clinical trials have suggested higher rates of achieving an R0 resection with the use of induction therapy for locally advanced TETs (9,11-16).
However, the variability in inclusion criteria and subjectivity in defining “marginally resectable” disease has limited the generalizability of this multimodal strategy (11,12,17). Further, several large multi-institutional database studies have described conflicting results, reporting worse long-term overall survival with the receipt of induction therapy (18), no difference in cancer-specific or recurrence-free survival (19), and no difference in tumor resectability (9). In addition, the patient withdrawal rates from proceeding with surgery after induction therapy range from 4.8% to as high as 38.1% (9,11,12,20). Accordingly, induction therapy may unnecessarily delay or impede upfront curative resection and predispose patients to disease progression (9).
Approaching the current literature describing induction therapies for TETs requires caution. Many of the available studies are retrospective, heterogeneous (include both thymomas and thymic carcinomas), and lack upfront surgical control cohorts (21). In addition, the published clinical trials are limited to single arm phase II studies (11,12,20). Using the existing literature, we will carefully review the role of induction therapy and attempt to identify when it may be appropriate to use. We present the following article in accordance with the NARRATIVE REVIEW reporting checklist (available at http://dx.doi.org/10.21037/med-20-20).
Utility of induction therapy
There remains no general consensus on the utility of administering induction therapy, and without randomized clinical trials, it will be difficult to provide definitive evidence to support it. However, several studies have attempted to shed light on the topic. The premise of employing induction therapy is based on the seminal work of two cooperative group clinical trials establishing the sensitivity of thymomas to chemotherapy, led by the Eastern Cooperative Oncology Group (ECOG) (22) and European Organization for Research and Treatment of Cancer (EORTC) (23) in patients with unresectable or metastatic disease (21). These trials specifically used cisplatin, doxorubicin, cyclophosphamide (CAP) and cisplatin-etoposide regimens, respectively. A number of studies of chemotherapy combinations, majority platinum-based, have since followed in cohorts that included a mix of patients with thymoma and thymic carcinoma (20,24-28).
In a meta-analysis comprised of 12 studies and a collective 286 patients, there was a pooled rate of response to induction therapy of 59%, complete resection rate of 73%, 5-year survival of 87%, and 10-year survival of 76% (29). The studies included in the aforementioned analyses consisted predominantly of Masaoka stage III and IVA thymic tumors (thymoma and thymic carcinoma), most often treated with induction chemotherapy alone. The favorable results reported in this analytic summary suggest that select patients with advanced stage TETs may benefit from induction therapy prior to surgical resection. Further, a number of retrospective case-series examining the oncologic benefits of induction therapy have reported promising results, showing clinical response rates ranging from 62% to 100% and complete resection rates ranging from 22% to 92% (13,15,16,21,30,31).
Only a few prospective clinical trials investigating induction therapy in locally advanced thymomas exist. Kim and colleagues utilized a CAP (cisplatin, doxorubicin, cyclophosphamide) + prednisone regimen, reporting 14% complete responses, 63% partial responses, and 77% 5-year disease free survival (11). Another phase II clinical trial using dose-dense cisplatin, vincristine, doxorubicin, etoposide (CODE) regimen reported no patients with a complete response, 62% with a partial response, and a 5-year progression-free survival of 43% (12). Amongst patients that proceeded to surgery in the aforementioned clinical trials, a complete resection rate was reported in 76% (11) and 69% (12) of patients.
Given the aggressive and invasive properties of thymic carcinoma, the utility of induction therapy for this particular histology may have the greatest potential benefit (32). The literature, however, is limited to a few case-series, which have reported encouraging results. In a single institutional experience from Osaka University Hospital, 16 patients with invasive disease involving the great vessels or metastatic disease to the mediastinal or intrathoracic lymph nodes received a platinum based neoadjuvant chemotherapy regimen, and in the vast majority of cases (75%) concurrent induction radiation therapy (33). This study demonstrated a 69% complete resection rate and 71% 5-year survival rate. Upon comparing long-term survival between patients with a complete and incomplete resection, a significant survival advantage was seen in the former (33). These were reassuring results given the known poor prognosis of patients with thymic carcinoma involving the great vessels (34). In another case-series of 7 patients with Masaoka stage III/IV thymic carcinoma treated with an induction CODE chemotherapy regimen followed by surgery, an impressive 85% complete resection rate was achieved, with a 10-year overall survival of 80% and 10-year relapse-free survival of 53.6% (35). The importance of a complete resection in patients with thymic carcinoma is supported by several studies, thus the role of using induction therapy as a means of rendering thymic carcinoma a surgically manageable disease to achieve an R0 resection is promising (36-39). However, it should be noted that these favorable outcomes are likely influenced by selection bias, as patients within these study populations may well be selected for good performance status and lower tumor burden (35).
Impact of induction therapy on R0 resection and stage
Achieving a surgically complete resection has long been reported to be the dominant prognostic factor for patients undergoing surgical resection of TETs (40-42). In this context, the likely primary oncologic benefit of induction therapy is reducing the thymic tumor burden to increase the likelihood of an R0 resection. In stark comparison to the near 100% rate of complete resection for Masaoka stage I thymomas, stage III and stage IV disease have average resectability rates of 47% and 26% respectively (6). These complete resection rates are noticeably lower than those reported in patients receiving induction therapy as discussed above. This data, however, remains only suggestive, because of the possibility of patient selection bias in these studies confounding the results. Further, radiographic response to induction therapy has failed to demonstrate any association with enhanced R0 resection rates (29). Interestingly, a small baseline pre-induction tumor volume has strongly been linked to achieving complete resection, however, post-induction volume has not shown the same association (43).
Apart from the hope of facilitating a complete resection, studies show conflicting results on whether or not induction therapy down-stages TETs in large numbers of patients. The data from the Chinese Alliance for Research in Thymomas (ChART) database demonstrated higher 5-year overall survival in patients receiving induction therapy (both thymomas and thymic carcinomas) that were down-staged; however, in a stage III only sub-analysis, there were equivalent 5-year survival rates to those who received upfront surgical resection (44). Other studies have reported no association between down-staging and disease-free or overall survival (9,45).
Role of tumor histology
Thymic carcinoma is predominantly distinguished from thymoma based on biological properties, morphologic appearance, prominent cytologic atypia, and clinical prognosis (25). Contrary to the indolent clinical course associated with thymomas, thymic carcinoma is often highly aggressive and typically diagnosed at an advanced stage. Accordingly, thymic carcinomas are less commonly considered to be completely resectable at presentation, and thus induction chemotherapy or combined chemoradiotherapy is often elected to attempt to render the disease more amenable to complete resection (46).
Historically, thymomas have been considered to be more sensitive to chemotherapy than thymic carcinomas, in part due to the lymphocytic effect of cytotoxic agents and steroids in select thymoma histotypes (24). However, several studies comparing the response rates for thymoma and thymic carcinoma have not demonstrated a clear advantage of either pathologic tumor types in terms of chemosensitivity (45,47-50). Three studies comparing the efficacy of a platinum-anthracycline regimen demonstrated superior response rates in patients with thymoma over those with thymic carcinoma (47,48,51), while another study reported a higher response rate in patients with thymic carcinoma (49). These variable trends in response rate to chemotherapy persist for a variety of other drug regimens as well (26,50,52,53).
Although the existing literature is conflicting, a recent systematic review of 55 eligible articles demonstrated response rates predominantly above 50%, independent of the line of treatment or histological type (24). These data demonstrating chemosensitivity of thymic carcinoma suggest that induction therapy may also be useful for select cases of locally advanced thymic carcinoma.
Multi-institutional investigations
Several large multi-institutional national databases have been retrospectively reviewed and have provided valuable insight into the utility of induction therapy, summarized in Table 1. The Japanese Association for Research on the Thymus (JART) examined patients with clinical Masaoka stage III thymomas undergoing induction therapy (primarily chemotherapy) and reported a 52.3% response rate. However, multivariable analysis revealed induction therapy to be associated with worse 10-year overall survival (HR 3.43, 95% CI: 1.85–7.37, P>0.001) (18). As acknowledged by the authors, the inherent selection biases of the study likely predisposed patients with more aggressive and invasive disease to have received induction therapy, thus limiting the conclusions that can be drawn (44). Confirming this bias, a substantial number of the patients receiving induction therapy were found to have radiographically larger tumors, multi-site disease, and a higher rate of phrenic nerve involvement than those stage III tumors that did not receive induction therapy (18).
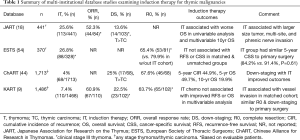
Full table
Another valuable international resource, the European Society of Thoracic Surgeons (ESTS) thymic database was used to examine multimodal therapy for advanced stage III thymomas (54). Slightly over a quarter of the study cohort received induction therapy. In comparative analyses, patients receiving induction therapy were more likely to be younger, have more aggressive World Health Organization (WHO) histologic type, and more often had an incomplete resection compared to those receiving surgery upfront. Patients receiving induction therapy, most often a platinum based chemotherapy regimen, did not demonstrate a cancer-specific survival (CSS) advantage when compared to upfront surgery in a propensity matched analysis (5-year CSS 84.2% vs. 91.4%, P=0.61) (54). Moreover, as with many of the large retrospective TET studies, a major limitation is the lack of data describing the indication for induction therapy (55). Further, this particular dataset lacked information on radiologic response and down-staging rates, so the true efficacy profile of induction therapy could not be measured.
The ChART database was used to examine trends in down-staging locally advanced clinical Masaoka stage III-IV TETs (44). Of the 1,714 patient records within the dataset, a mere 4% received preoperative induction therapy. Given the extensive span of data from 1994–2012, temporal trends in induction therapy were noted. There were no statistical differences in the utilization of induction therapy across the course of the study. There was, however, an increased use of chemotherapy or radiation alone and decreased use of combined chemoradiation. On pathologic examination, 25% of the recipients of induction therapy were down-staged (44). Stratified by histologic type, significantly more thymomas were down-staged than thymic carcinomas and thymic carcinoid tumors. In a sub-analysis, recipients of induction therapy with substantial responses resulting in down-staging had significantly higher 5-year overall survival than those tumors that were not down-staged (93.8% vs. 35.6%, P=0.0013) (44).
The most recent multi-institutional study comes from the Korean Association for Research on the Thymus database (KART), in which patients receiving induction chemotherapy were compared to those receiving upfront surgery (9). In this propensity score-matched analysis, patients receiving induction therapy required more frequent blood transfusions, had higher blood loss, and longer operative times than those receiving upfront surgery, yet there were no differences between the two cohorts in terms of postoperative mortality, postoperative complications, or length of stay. Further, there were no differences in pathologically complete resections. Nor was there a difference in 5-year overall survival or 3-year recurrence-free survival rates (9).
Over the last decade there have been multiple collaborative efforts on the regional, national, and international fronts that have challenged historical dogma and provided invaluable information in guiding treatment for patients with thymic malignancies (56). Nevertheless, in terms of the value of induction therapy, a clear consensus still cannot be established based on the aforementioned multi-institutional studies. These large retrospective database studies do, however, provide a stepping stone for future randomized controlled studies.
Combining chemotherapy and radiotherapy
The synergistic advantage of platinum based chemotherapy and radiation has been well established in thoracic oncology, particularly lung cancer, and this combination has generally been well tolerated (57,58). Using this premise, Wright and colleagues hypothesized that concurrent chemoradiotherapy would permit the most effective induction for TETs and accordingly result in more complete resections and improved survival (59). In this group’s proof of concept study, induction therapy [cisplatin/etoposide + radiation (target dose 40–45 Gy)] for locally advanced TETs (thymoma and thymic carcinoma) was well tolerated, with favorable survival profiles (59). Based on these preliminary results, a multi-institutional, prospective, phase II clinical trial examined the pathologic response to this induction therapy (17). Among 21 patients completing induction therapy, none had a complete pathologic response, however, 24% had <10% viable tumor remaining (17). In this study the vast majority of patients who had a near complete response had thymic carcinoma, suggesting patients with higher risk WHO histologic types may respond better to a concurrent chemoradiotherapy treatment plan. Furthermore, 77% (17 of 22) underwent an R0 resection (17). By comparison, large prospective clinical trials examining induction chemotherapy alone for thymoma reported a complete resection rate of 43% (9 of 21) (12) and 76% (16 of 21) (11). However, direct comparison of these induction therapy studies with and without the addition of radiation cannot be made given the inconsistent inclusion criteria, histologic subtype distribution, and differing chemotherapy regimens between the studies (17).
Johnson and colleagues investigated the radiographic and histologic response in 49 patients with TETs to induction therapy, comparing chemotherapy, chemoradiation, and radiation alone followed by surgery in a single institution retrospective study (60). The authors identified chemoradiotherapy as having the highest response and lowest percent of remaining viable tumor cells, whereas those receiving neoadjuvant radiation alone had the worst response. Additionally, the study demonstrated the importance of tumor histologic type, revealing greater pathologic responses with any type of induction therapy in thymic carcinomas than thymomas (60).
These available small studies reporting the use of induction chemoradiation have at least comparable, and perhaps higher, response rates compared to induction chemotherapy alone. However, a caveat that must be considered is the toxicity associated with a combined modality induction treatment (21). In the aforementioned phase II clinical trial of chemoradiotherapy, treatment related complications occurred in 41% in the intention to treat analysis (17). Complications ranged from dehydration to cardiac arrest. Beyond the early potential increase in adverse events, radiotherapy is also well-known to contribute over years to the risk of coronary and valvular disease (21), therefore, it is difficult to advocate for adding radiotherapy to induction chemotherapy.
Patient selection
As there are no randomized clinical trials to support or oppose the use of induction therapy, medical providers are relegated to using the data that currently exists through the above-described single institutional experiences, large organizational databases, and phase II clinical trials.
The ultimate decision generally falls to the assessment of the invasiveness of the thymic tumor, and the surgeon’s opinion of the likelihood of being able to achieve a complete resection (54). Since there is morbidity associated with induction therapy and occasional cases that may never come to resection as a result of treatment-related complications or disease progression, if there is confidence in a high likelihood of complete resection, upfront surgical resection should be performed.
Determining whether a complete resection will be possible, however, is not an easy task, even for highly experienced thoracic surgeons. Therefore, the ability to identify objective preoperative tumor imaging characteristics that may predict resectability would be a major advance and would aid in the clinical decision-making process of whether or not to utilize induction therapy. Analysis of the JART database demonstrated lower rates of complete resection in patients with thymic tumor invading the great vessels when compared to invasion of the pericardium and lung (18). In fact, resection of the pericardium and small wedges of the adjacent lung adds essentially no morbidity to a thymic resection, and nearly always achieves negative margins in those areas. Resection of great vessels on the other hand, is far more complex and morbid, and surgeons outside of high volume centers may hesitate to undertake these.
Hayes and colleagues also identified preoperative computed tomography (CT) imaging characteristics that predicted an incomplete resection for thymomas, including ≥50% abutment of the circumference of an adjacent vessel and pleural nodularity (61). Other radiographic features noted to be associated with advanced stages of TETs include tumor size ≥7 cm, lobulated tumor contour, infiltration of mediastinal fat, and elevated hemidiaphragm (62,63). On the contrary, there is controversy in the size threshold predicting for incomplete resection status or advanced stage, as some larger thymomas can be non-invasive and completely resected upfront in asymptomatic patients (64). The need for resection of a phrenic nerve is difficult to predict based on imaging in the absence of an elevated hemidiaphragm, and remains a challenge for surgeons.
Understanding these high-risk features that may impede a complete resection should help providers appropriately select patients that may benefit from induction therapy. Certainly, the selection of candidates for induction therapy (either chemotherapy alone, or chemoradiotherapy) should take place in a multidisciplinary tumor board including medical oncologist, surgeon, and radiation oncologist with experience managing advanced thymic malignancies (21).
Conclusions
There are currently no data that can allow one to definitively support or refute the use of induction therapy prior to resection of locally advanced thymic malignancies. Without randomized controlled trials, it is unlikely the thymic medical community will arrive at a consensus on its utility. Based on the existing retrospective case-series, multi-institutional investigations, and prospective phase II trials, it appears that induction therapy likely has a benefit in carefully selected patients with marginally resectable disease. Specific tumor characteristics and extent of tumor invasion of advanced thymic malignancies should certainly prompt multidisciplinary discussion in which the options of induction therapy vs. primary surgical resection are weighed on a case by case basis. The primary objective of considering induction therapy should be facilitating a complete resection; other endpoints such as down-staging or pathologic response have not been shown to result in meaningful improvements in long-term outcomes.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Mirella Marino, Brett W. Carter) for the series “Dedicated to the 10th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2019)” published in Mediastinum. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Reporting Checklist: The authors have completed the NARRATIVE REVIEW reporting checklist. Available at http://dx.doi.org/10.21037/med-20-20
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-20). The series “Dedicated to the 10th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2019)” was commissioned by the editorial office without any funding or sponsorship. SKP reports grants from Epicentrx, Bayer, Boehringer Ingelheim, 47 Inc., personal fees from Pfizer, personal fees and other from AstraZeneca, and personal fees from G1 Therapeutic, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Huang J, Detterbeck FC, Wang Z, et al. Standard outcome measures for thymic malignancies. J Thorac Oncol 2011;6:S1691-7. [Crossref] [PubMed]
- de Jong WK, Blaauwgeers JL, Schaapveld M, et al. Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur J Cancer 2008;44:123-30. [Crossref] [PubMed]
- Loehrer PJ Sr, Wang W, Johnson DH, et al. Octreotide alone or with prednisone in patients with advanced thymoma and thymic carcinoma: an Eastern Cooperative Oncology Group Phase II Trial. J Clin Oncol 2004;22:293-9. [Crossref] [PubMed]
- Rajan A, Giaccone G. Treatment of advanced thymoma and thymic carcinoma. Curr Treat Options Oncol 2008;9:277-87. [Crossref] [PubMed]
- Kelly RJ, Petrini I, Rajan A, et al. Thymic malignancies: from clinical management to targeted therapies. J Clin Oncol 2011;29:4820. [Crossref] [PubMed]
- Detterbeck FC, Parsons AM. Thymic tumors. Ann Thorac Surg 2004;77:1860-9. [Crossref] [PubMed]
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: Proposal for an Evidence-Based Stage Classification System for the Forthcoming (8th) Edition of the TNM Classification of Malignant Tumors. J Thorac Oncol 2014;9:S65-S72. [Crossref] [PubMed]
- Ahmad U, Yao X, Detterbeck F, et al. Thymic carcinoma outcomes and prognosis: Results of an international analysis. J Thorac Cardiovasc Surg 2015;149:95-100, 101.e1-2. [Crossref] [PubMed]
- Park S, Park IK, Kim YT, et al. Comparison of Neoadjuvant Chemotherapy Followed by Surgery to Upfront Surgery for Thymic Malignancy. Ann Thorac Surg 2019;107:355-62. [Crossref] [PubMed]
- Giaccone G, Musella R, Bertetto O, et al. Cisplatin-containing chemotherapy in the treatment of invasive thymoma: report of five cases. Cancer Treat Rep 1985;69:695-7. [PubMed]
- Kim ES, Putnam JB, Komaki R, et al. Phase II study of a multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation therapy, and consolidation chemotherapy for unresectable malignant thymomas. Lung Cancer 2004;44:369-79. [Crossref] [PubMed]
- Kunitoh H, Tamura T, Shibata T, et al. A phase II trial of dose-dense chemotherapy, followed by surgical resection and/or thoracic radiotherapy, in locally advanced thymoma: report of a Japan Clinical Oncology Group trial (JCOG 9606). Br J Cancer 2010;103:6-11. [Crossref] [PubMed]
- Lucchi M, Ambrogi MC, Duranti L, et al. Advanced stage thymomas and thymic carcinomas: results of multimodality treatments. Ann Thorac Surg 2005;79:1840-4. [Crossref] [PubMed]
- Venuta F, Rendina EA, Longo F, et al. Long-term outcome after multimodality treatment for stage III thymic tumors. Ann Thorac Surg 2003;76:1866-72. [Crossref] [PubMed]
- Rea F, Sartori F, Loy M, et al. Chemotherapy and operation for invasive thymoma. J Thorac Cardiovasc Surg 1993;106:543-9. [Crossref] [PubMed]
- Macchiarini P, Chella A, Ducci F, et al. Neoadjuvant chemotherapy, surgery, and postoperative radiation therapy for invasive thymoma. Cancer 1991;68:706-13. [Crossref] [PubMed]
- Korst RJ, Bezjak A, Blackmon S, et al. Neoadjuvant chemoradiotherapy for locally advanced thymic tumors: a phase II, multi-institutional clinical trial. J Thorac Cardiovasc Surg 2014;147:36-44, 46.e1. [Crossref] [PubMed]
- Yamada Y, Yoshino I, Nakajima J, et al. Surgical Outcomes of Patients With Stage III Thymoma in the Japanese Nationwide Database. Ann Thorac Surg 2015;100:961-7. [Crossref] [PubMed]
- Leuzzi G, Alessandrini G, Sperduti I, et al. Induction therapy versus initial surgery in advanced thymic tumors: Perioperative and oncological outcome. Thorac Cardiovasc Surg 2017;65:234-43. [PubMed]
- Park S, Ahn M-j, Ahn JS, et al. A prospective phase II trial of induction chemotherapy with docetaxel/cisplatin for Masaoka stage III/IV thymic epithelial tumors. J Thorac Oncol 2013;8:959-66. [Crossref] [PubMed]
- Ahmad U, Huang J. Induction Therapy for Thymoma. Thorac Surg Clin 2016;26:325-32. [Crossref] [PubMed]
- Loehrer PJ Sr, Kim K, Aisner SC, et al. Cisplatin plus doxorubicin plus cyclophosphamide in metastatic or recurrent thymoma: final results of an intergroup trial. The Eastern Cooperative Oncology Group, Southwest Oncology Group, and Southeastern Cancer Study Group. J Clin Oncol 1994;12:1164-8. [Crossref] [PubMed]
- Giaccone G, Ardizzoni A, Kirkpatrick A, et al. Cisplatin and etoposide combination chemotherapy for locally advanced or metastatic thymoma. A phase II study of the European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol 1996;14:814-20. [Crossref] [PubMed]
- Berghmans T, Durieux V, Holbrechts S, et al. Systemic treatments for thymoma and thymic carcinoma: A systematic review. Lung Cancer 2018;126:25-31. [Crossref] [PubMed]
- Okuma Y, Hosomi Y, Takagi Y, et al. Clinical outcomes with chemotherapy for advanced thymic carcinoma. Lung Cancer 2013;80:75-80. [Crossref] [PubMed]
- Grassin F, Paleiron N, André M, et al. Combined etoposide, ifosfamide, and cisplatin in the treatment of patients with advanced thymoma and thymic carcinoma. A French experience. J Thorac Oncol 2010;5:893-7. [Crossref] [PubMed]
- Schmitt J, Loehrer PJ Sr. The role of chemotherapy in advanced thymoma. J Thorac Oncol 2010;5:S357-60. [Crossref] [PubMed]
- Oguri T, Achiwa H, Kato D, et al. Efficacy of docetaxel as a second-line chemotherapy for thymic carcinoma. Chemotherapy 2004;50:279-82. [Crossref] [PubMed]
- Hamaji M, Ali SO, Burt BM. A meta-analysis of induction therapy for advanced thymic epithelial tumors. Ann Thorac Surg 2015;99:1848-56. [Crossref] [PubMed]
- Berruti A, Borasio P, Roncari A, et al. Neoadjuvant chemotherapy with adriamycin, cisplatin, vincristine and cyclophosphamide (ADOC) in invasive thymomas: results in six patients. Ann Oncol 1993;4:429-31. [Crossref] [PubMed]
- Yokoi K, Matsuguma H, Nakahara R, et al. Multidisciplinary Treatment for Advanced Invasive Thymoma with Cisplatin, Doxorubicin, and Methylprednisolone. J Thorac Oncol 2007;2:73-8. [Crossref] [PubMed]
- Girard N, Lal R, Wakelee H, et al. Chemotherapy definitions and policies for thymic malignancies. J Thorac Oncol 2011;6:S1749-55. [Crossref] [PubMed]
- Shintani Y, Inoue M, Kawamura T, et al. Multimodality treatment for advanced thymic carcinoma: outcomes of induction therapy followed by surgical resection in 16 cases at a single institution. Gen Thorac Cardiovasc Surg 2015;63:159-63. [Crossref] [PubMed]
- Tseng YL, Wang ST, Wu MH, et al. Thymic carcinoma: involvement of great vessels indicates poor prognosis. Ann Thorac Surg 2003;76:1041-5. [Crossref] [PubMed]
- Kawasaki H, Taira N, Ichi T, et al. Weekly chemotherapy with cisplatin, vincristine, doxorubicin, and etoposide followed by surgery for thymic carcinoma. Eur J Surg Oncol 2014;40:1151-5. [Crossref] [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84. [Crossref] [PubMed]
- Yano M, Sasaki H, Yokoyama T, et al. Thymic carcinoma: 30 cases at a single institution. J Thorac Oncol 2008;3:265-9. [Crossref] [PubMed]
- Filosso PL, Guerrera F, Rendina AE, et al. Outcome of surgically resected thymic carcinoma: a multicenter experience. Lung Cancer 2014;83:205-10. [Crossref] [PubMed]
- Lee CY, Bae MK, Park IK, et al. Early Masaoka stage and complete resection is important for prognosis of thymic carcinoma: a 20-year experience at a single institution. Eur J Cardiothorac Surg 2009;36:159-62; discussion 163. [Crossref] [PubMed]
- Moser B, Scharitzer M, Hacker S, et al. Thymomas and thymic carcinomas: prognostic factors and multimodal management. Thorac Cardiovasc Surg 2014;62:153-60. [PubMed]
- Roden AC, Eunhee SY, Jenkins SM, et al. Modified Masaoka stage and size are independent prognostic predictors in thymoma and modified Masaoka stage is superior to histopathologic classifications. J Thorac Oncol 2015;10:691-700. [Crossref] [PubMed]
- Schneider PM, Fellbaum C, Fink U, et al. Prognostic importance of histomorphologic subclassification for epithelial thymic tumors. Ann Surg Oncol 1997;4:46-56. [Crossref] [PubMed]
- Suh JW, Park SY, Lee CY, et al. Neoadjuvant therapy for thymic neoplasms reduces tumor volume per 3D-reconstructed images but does not improve the complete resection rate. PLoS One 2019;14:e0214291 [Crossref] [PubMed]
- Wei Y, Gu Z, Shen Y, et al. Preoperative induction therapy for locally advanced thymic tumors: a retrospective analysis using the ChART database. J Thorac Dis 2016;8:665-72. [Crossref] [PubMed]
- Cardillo G, Lucchi M, Marulli G, et al. Induction therapy followed by surgical resection in Stage-III thimic epithelial tumors: Long-term results from a multicentre analysis of 108 cases. Lung Cancer 2016;93:88-94. [Crossref] [PubMed]
- Syrios J, Diamantis N, Fergadis E, et al. Advances in thymic carcinoma diagnosis and treatment: a review of literature. Med Oncol 2014;31:44. [Crossref] [PubMed]
- Liu J, Wang L, Huang M, et al. Topoisomerase 2α plays a pivotal role in the tumor biology of stage IV thymic neoplasia. Cancer 2007;109:502-9. [Crossref] [PubMed]
- Thomas A, Rajan A, Szabo E, et al. A phase I/II trial of belinostat in combination with cisplatin, doxorubicin, and cyclophosphamide in thymic epithelial tumors: a clinical and translational study. Clin Cancer Res 2014;20:5392-402. [Crossref] [PubMed]
- Inoue A, Sugawara S, Harada M, et al. Phase II study of Amrubicin combined with carboplatin for thymic carcinoma and invasive thymoma: North Japan Lung Cancer group study 0803. J Thorac Oncol 2014;9:1805-9. [Crossref] [PubMed]
- Kim HS, Lee JY, Lim SH, et al. A prospective phase II study of cisplatin and Cremophor EL-free paclitaxel (Genexol-PM) in patients with unresectable thymic epithelial tumors. J Thorac Oncol 2015;10:1800-6. [Crossref] [PubMed]
- Cardillo G, Carleo F, Giunti R, et al. Predictors of survival in patients with locally advanced thymoma and thymic carcinoma (Masaoka stages III and IVa). Eur J Cardiothorac Surg 2010;37:819-23. [Crossref] [PubMed]
- Loehrer PJ Sr, Jiroutek M, Aisner S, et al. Combined etoposide, ifosfamide, and cisplatin in the treatment of patients with advanced thymoma and thymic carcinoma: an intergroup trial. Cancer 2001;91:2010-5. [Crossref] [PubMed]
- Lemma GL, Lee JW, Aisner SC, et al. Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma. J Clin Oncol 2011;29:2060. [Crossref] [PubMed]
- Leuzzi G, Rocco G, Ruffini E, et al. Multimodality therapy for locally advanced thymomas: A propensity score–matched cohort study from the European Society of Thoracic Surgeons Database. J Thorac Cardiovasc Surg 2016;151:47-57. e1.
- Girard N, du Vignaux CM. How large databases may impact clinical practices for rare tumors—postoperative chemotherapy in thymic malignancies. J Thorac Dis 2016;8:1863. [Crossref] [PubMed]
- Ruffini E, Filosso PL, Guerrera F, et al. Optimal surgical approach to thymic malignancies: New trends challenging old dogmas. Lung Cancer 2018;118:161-70. [Crossref] [PubMed]
- Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for superior sulcus non–small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Clin Oncol 2007;25:313-8. [Crossref] [PubMed]
- Choi NC, Carey RW, Daly W, et al. Potential impact on survival of improved tumor downstaging and resection rate by preoperative twice-daily radiation and concurrent chemotherapy in stage IIIA non-small-cell lung cancer. J Clin Oncol 1997;15:712-22. [Crossref] [PubMed]
- Wright CD, Choi NC, Wain JC, et al. Induction chemoradiotherapy followed by resection for locally advanced Masaoka stage III and IVA thymic tumors. Ann Thorac Surg 2008;85:385-9. [Crossref] [PubMed]
- Johnson GB, Aubry MC, Yi ES, et al. Radiologic Response to Neoadjuvant Treatment Predicts Histologic Response in Thymic Epithelial Tumors. J Thorac Oncol 2017;12:354-67. [Crossref] [PubMed]
- Hayes SA, Huang J, Plodkowski AJ, et al. Preoperative computed tomography findings predict surgical resectability of thymoma. J Thorac Oncol 2014;9:1023-30. [Crossref] [PubMed]
- Marom EM, Milito MA, Moran CA, et al. Computed tomography findings predicting invasiveness of thymoma. J Thorac Oncol 2011;6:1274-81. [Crossref] [PubMed]
- Padda SK, Terrone D, Tian L, et al. Computed Tomography Features associated with 8th Edition TNM Stage Classification for Thymic Epithelial Tumors. J Thorac Imaging 2018;33:176.
- Burt BM, Yao X, Shrager J, et al. Determinants of complete resection of thymoma by minimally invasive and open thymectomy: analysis of an international registry. J Thorac Oncol 2017;12:129-36. [Crossref] [PubMed]
Cite this article as: Patel DC, Shrager JB, Padda SK. The role of induction therapy for thymic malignancies: a narrative review. Mediastinum 2020;4:36.


