Vascular tumors of the mediastinum
Intermediate (locally aggressive)
Kaposiform hemangioendothelioma (KHE)
Clinical features
Originally described as a deep-seated tumor of infants, as reflected in its archaic name kaposiform infantile hemangioendothelioma, this lesion is now known to affect a range of ages and a variety of sites (1). In a cohort of 146 patients with KHE, the vast majority were diagnosed within their first year of life, and half of the cases involved the extremities (2). Adults tend to suffer worse outcomes (3). The mediastinum, including the heart, is a known site of origin (4-7). Morbidity stems from complications of: (I) locally aggressive tumor growth, resulting in compression of vital structures, and/or (II) platelet trapping within the vascular channels of the tumor, leading to consumptive coagulopathy, a condition known as the eponymous Kasabach-Merritt phenomenon. During the active phase of the Kasabach-Merritt phenomenon, the size of the tumor mass increases as clotting progresses, further compressing vital structures. Consensus guidelines for treatment are emerging (8), with increasing evidence for the efficacy of mTOR inhibitor sirolimus (6,9).
Pathologic features
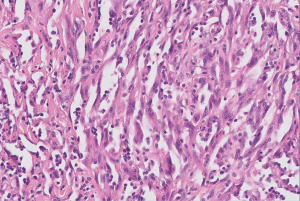
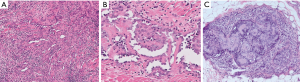
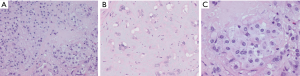
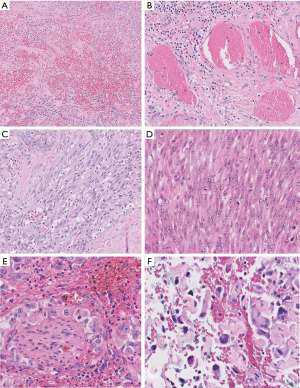
Low-power examination reveals an infiltrative yet lobular proliferation of cellular nodules composed of well formed capillaries admixed with short fascicles of spindle cells with slit-like vascular channels (Figure 1). Crescentic lymphatic channels reside at the periphery of nodules. Extravasated RBCs, intraluminal thrombi, and hemosiderin deposits can also be observed (9,10).
Ancillary studies
Focal expression of lymphatic markers (D2-40, PROX1, and VEGFR3), and more extensively, vascular markers (CD31, CD34, ERG), is observed in the spindled endothelial cells of KHE (Figure 1C) (9). There is no expression of human herpesvirus 8 (HHV8). Based on limited molecular data, there is some evidence of a translocation between chromosomes 13 and 16, while other cases exhibit an activating mutation of GNA14 (a paralogue of GNAQ) (9).
Differential diagnosis
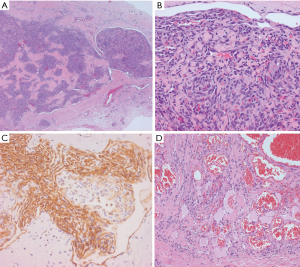
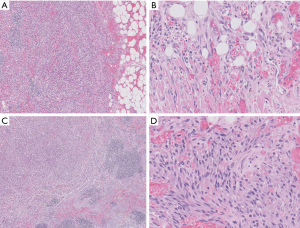
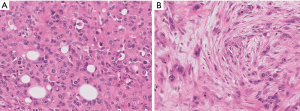
Morphologic resemblance to Kaposi sarcoma (KS) is obvious from the name of the tumor. However, the consistently lobular growth pattern of KHE is not a feature of KS, and HHV8 expression is absent in KHE. Infantile (juvenile) hemangioma may enter the differential diagnosis owing to a lobulated tumor border together with densely packed vascular nodules (Figure 2), but these tumors spontaneously involute, are not associated with the Kasabach-Merritt phenomenon, and express GLUT-1 by immunohistochemistry (11).
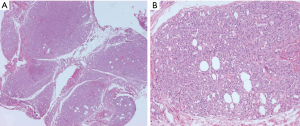
Kaposiform lymphangiomatosis (KLA) is a recently introduced term for a lymphatic anomaly which overlaps substantially with KHE and may in fact represent a clinicopathologic variant of KHE rather than a disease sui generis. To further confuse the picture, the terms “generalized lymphatic anomaly and Gorham-Stout disease” have entered into this mix of lymphatic anomalies (12,13). For convenience, one can regard these as clinical variants of histologically similar lymphatic pathologies. A few subtle differences can be identified between KHE and KLA: (I) multifocality within the mediastinum and lung is more characteristic of KLA, (II) histologically, KLA exhibits more dispersed and poorly defined collections of tumor cells (Figure 3), and (III) both KLA and KHE have increased activity in RAS/PI3K/mTOR signaling pathways, but somatic activating NRAS variants have been found in KLA but not KHE (14).
Intermediate (rarely metastasizing)
Retiform hemangioendothelioma
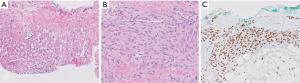
Cases of retiform hemangioendothelioma involving mediastinal structures are few and far between; therefore, only a brief description follows. This tumor most commonly involves superficial tissue planes. A mediastinal case presented as pleural nodules in a patient with dry cough, dyspnea, and pleural effusion (15).
Histologic examination discloses arborizing vascular channels recapitulating the structure of the rete testis (Figure 4). The vascular channels are lined by hobnail endothelial cells. Numerous lymphocytes permeate the channels and the stroma. Intravascular papillae with hyaline cores may be seen, creating overlap with papillary intralymphatic angioendothelioma (Dabska tumor) (Figure 4C). Endothelial cells express vascular markers and lymphatic marker PROX1, but they only rarely express D2-40 (16).
Papillary intralymphatic angioendothelioma (Dabska tumor)
Reports of this tumor in the mediastinum were not found, but since cases have been reported in intra-abdominal sites and even within the bone (17), the thorax could theoretically be involved. The key to diagnosing this tumor lies in recognizing the distinctive hobnail, “match-stick”-like appearance of the endothelial cells lining the lymphatic channels and covering the papillary hyaline cores. Immunohistochemistry shows immunoreactivity for the lymphatic markers (D2-40, PROX1, and VEGFR3). There is significant overlap with retiform hemangioendothelioma (discussed above).
Composite hemangioendothelioma
By definition, this tumor exhibits at least two morphologically distinct vascular components (18). A lone report describes mediastinal involvement (19). Akin to retiform hemangioendothelioma, this tumor most commonly involves superficial tissue planes. There is an association with Maffucci syndrome and Kasabach-Merritt phenomenon (20), though the latter is most closely linked to KHE. These tumors usually behave in a low-grade manner, with potential for local recurrence but minimal potential for distant metastasis and death (20,21). While the combination of retiform hemangioendothelioma and epithelioid hemangioendothelioma (EHE) is most common, many other benign and malignant components can be observed, ranging from hemangioma to angiosarcoma-like areas (18). Rarely, there are areas with a neuroendocrine appearance which show synaptophysin immunoreactivity; this may portend a worse prognosis. Molecular studies have uncovered novel PTBP1-MAML2 and EPC1-PHC2 gene fusions in two cases (22). Despite the rarity of these neuroendocrine cases, the 5th Edition WHO Classification formally recognizes them as a subtype of composite hemangioendothelioma (18).
Kaposi sarcoma (KS)
Clinical features
Typically a tumor of mucocutaneous and visceral sites, KS can affect any body site, the mediastinum being no exception. Lymph nodes are frequently involved (23). KS manifests as one of several clinical subtypes: (I) classic (indolent), (II) endemic (African), (III) AIDS-associated, and (IV) iatrogenic (18). Cardiac KS has been found across all clinical subdivisions, based on autopsy findings between the 1950s through the 1980s. The epicardium seems to be more frequently affected than the myocardium or endocardium (23). KS can also involve the thoracic duct and adjacent mediastinal structures, manifesting as chylothorax. The thoracic duct can serve as either a site of origin or a target of metastasis for KS (23,24). Doxorubicin is frequently the first-line therapy for systemic or extensive KS, but newer targeted therapies are showing promise in clinical trials (25).
Pathologic features
Characteristic features include relatively monotonous-appearing spindle cells, increased numbers of slit-like vascular channels, hemorrhage, hemosiderin deposition, and hyaline globules (Figure 5). If identified, the so-called “promontory sign” is a helpful feature, consisting of small vessels protruding into the lumen of a larger vessel. Chronic inflammation, including plasma cells, is a secondary feature. Many of the morphologic variants of KS presented in the cutaneous literature are not relevant to the mediastinum; however, brief mention will be made of the hemangioma-like, lymphangioma-like, and anaplastic variants (25,26). The hemangioma-like and lymphangioma-like patterns exhibit typical KS sarcoma in at least part of the lesion. Anaplastic KS is a pauci-vascular spindle cell proliferation with a greater degree of nuclear atypia, increased mitotic activity (5 to 20 mitoses per 10 high-power fields), and occasional necrosis. These features have been associated with aggressive behavior (27,28).
Ancillary studies
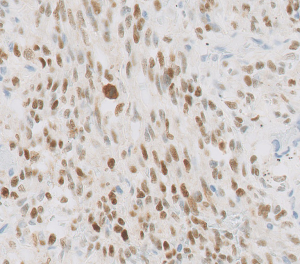
Tumor cells express vascular and lymphatic markers (29). Since HHV8 underlies KS tumorigenesis, HHV8 immunohistochemistry serves as the diagnostic key (Figure 6). Expression of the HHV8 virus produces the latency-associated nuclear antigen (LANA), which binds to retinoblastoma protein and p53, affecting cell proliferation and apoptosis (30). Comparative genomic hybridization studies have demonstrated 11q13 involvement, including FGF3/4 target genes. KRAS and TP53 aberrations have also been reported (18).
Differential diagnosis
Diagnosing lymphangioma/hemangioma-like KS requires a careful search to identify any areas of typical KS to trigger HHV8 immunohistochemistry. Distinction of anaplastic KS from angiosarcoma can be problematic, especially in spindle cell examples. The differential diagnosis with KHE was discussed above.
Pseudomyogenic hemangioendothelioma
Clinical features
An example of this relatively recently characterized tumor has been reported to involve the mediastinum; specifically, thoracic vertebrae with extension into the pleural cavity (31). A far more common presentation is a young man with multifocal involvement of tissue planes of the lower limb (cutaneous/subcutaneous > intramuscular > bone), or less commonly the trunk, upper limbs, or head and neck (18). Biological behavior is considered intermediate owing to frequent recurrences but low rates of distant metastasis (32).
Pathologic features
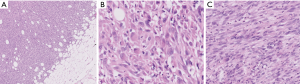
Microscopic examination reveals loose fascicles and sheets of plump spindled to polygonal cells with deep eosinophilic cytoplasm, resembling rhabdomyoblasts (Figure 7). Neutrophils are often present (Figure 7C). Nuclear pleomorphism tends to be limited, and mitotic activity is low (1 per 10 HPF on average). Vascular invasion and necrosis are infrequent features (32).
Ancilllary studies
Immunohistochemistry of the tumor reveals partial expression of both epithelial and vascular markers, characterized by immunoreactivity for AE1/AE3, ERG, and FLI1, but only variable Cam5.2 and CD31, and no expression of CD34 or MNF116 (32,33). The tumor is designated as pseudomyogenic because it lacks expression of myogenic marker desmin (and only variably expresses SMA) despite the myogenic/rhabdoid appearances. Strong and diffuse expression of FOSB by immunohistochemistry is effective in distinguishing pseudomyogenic hemangioendothelioma from morphologic simulants. FOSB immunoreactivity reflects a SERPINE1-FOSB (or ACTB-FOSB) gene fusion that has been identified as a consistent genetic alteration in these tumors (22,34,35). The fusion type has no bearing on clinical behavior, but the ACTB-FOSB variant has a greater tendency to present as a solitary lesion (36).
Differential diagnosis
The predominantly myoid-appearing spindle cell morphology, expression of ERG/FLI1, lack of EMA, CD34, and PAN-K expression, and intact INI-1 sets this tumor apart from epithelioid sarcoma, with which it is closely intertwined due to its original designation as “epithelioid sarcoma-like hemangioendothelioma” (32). Other spindle cell tumors that may enter the differential, such as sarcomatoid carcinoma and rhabdomyosarcoma, exhibit overt features of malignancy. Vascular tumors would only be considered upon observing FLI1/ERG immunoreactivity, as vasoformation is lacking by H&E examination. However, no other vascular tumor exhibits the unique constellation of morphologic and immunophenotypic traits of pseudomyogenic hemangioendothelioma.
Malignant
Epithelioid hemangioendothelioma (EHE)
Clinical features
EHE is a malignant vascular tumor composed of epithelioid endothelial cells in a myxohyaline matrix. Most commonly, it occurs in the somatic soft tissues, lung, and liver, but the tumor can arise from any body site. Adults are affected far more often than children. Superficial lesions are usually solitary, while visceral and bone tumors are frequently multiple, representing local metastatic spread (18). Mediastinal cases of EHE have earned a special place in the literature (37,38). Frequently, the tumor involves or originates from the superior vena cava or the innominate vein (39). For mediastinal cases, the size of the tumor is recorded in place of TNM staging (18). Several factors come into play for prognosticating these tumors: (I) anatomic site (lung and bone tumors behave worse than soft tissue ones), (II) select gross and microscopic tumor characteristics (tumor size and mitotic activity), and (III) molecular fusion type (discussed below). Pleural involvement portends an aggressive clinical course, with only 22% of patients surviving to 5 years, compared to a >70% overall survival rate (40).
Pathologic features
Angiocentric tumors expand the vessel wall, obliterate the lumen, and spread centrifugally into surrounding tissues (18). An infiltrative border is characteristic. The tumor consists of epithelioid (and sometimes fusiform) cells arranged as cords or nests within a hyaline (eosinophilic) to myxochondroid (basophilic) matrix (Figure 8). Primitive vasoformation manifests as intracytoplasmic vacuoles, which occasionally contain erythrocytes. Cells have round nuclei, inconspicuous nucleoli, intracytoplasmic vacuoles, and moderate amounts of eosinophilic cytoplasm. Cytologic atypia, mitotic activity, and necrosis are unusual (Figure 9), and their presence has been correlated with more aggressive behavior by some authors (40). However, Deyrup et al. have reported that cytologic atypia and tumor necrosis were not significant, but that >3 mitotic figures/50 high power fields and tumor size >3.0 cm correlated with unfavorable clinical outcomes (41).
Ancillary studies
Tumor cells express vascular markers (Figure 10) and occasionally epithelial markers; in fact, 29% of EHE show keratin expression (37). Molecular analysis reveals a CAMTA1-WWTR1 gene fusion in most cases (~90%), while a subset exhibits YAP1-TFE3 gene fusions (42). Alternative WWTR1 partners, MAML2 and ACTL6A, have been found to have a predilection for cardiac involvement (43). The YAP1-TFE3 subset bears several noteworthy differences relative to the CAMTA1-WWTR1 group: (I) more favorable outcomes (the 5-year survival being 86%, versus 59% for cases with CAMTA1-WWTR1 fusions) (40), (II) occurrence in a younger age group (44), (III) more solid growth, greater vasoformation, and brightly eosinophilic cytoplasm (45). These cases also exhibit nuclear TFE3 immunolabeling (Figure 10C) (44), albeit with imperfect specificity. CAMTA1 immunohistochemistry (Figure 11) is a useful surrogate for molecular testing (46).
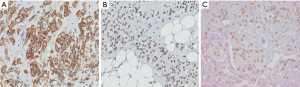
Differential diagnosis
In the mediastinum, where serous membranes abound, mesothelioma is a legitimate contender for the diagnosis of any malignant epithelioid neoplasm. The combination of any vasoformation, strong expression of vimentin (Figure 12), minimal cytokeratin expression, and expression of two or more endothelial markers clinches the diagnosis of EHE over mesothelioma (47). WT1 immunohistochemistry shows nuclear labeling of mesothelioma, while cytoplasmic labeling is seen in vascular tumors. Cytologically atypical cases of EHE are distinguished from epithelioid angiosarcoma based on the presence of distinctive extracellular matrix, intracytoplasmic vacuoles, and nuclear cytoplasmic inclusions (37). Clear-cut vasoformation with hemorrhage and formation of blood lakes should steer away from the diagnosis of EHE. Overall, angiosarcoma exhibits greater pleomorphism, mitotic activity, and frequent necrosis. On limited biopsy samples, when the distinction is difficult, molecular studies can provide a solution. However, some literature indicates that all epithelioid vascular tumors of the pleura should be considered highly malignant regardless of EHE-like histologic features (48). This opinion is consistent with the results of recent studies showing poor outcomes in cases with pleural involvement (mentioned above). For cases arising from veins, consideration may be given to intravascular epithelioid hemangioma, but these tumors exhibit lobular/multilobular growth rather than the infiltrative cords and strands of tumor cells seen in EHE (49).
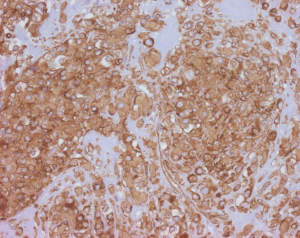
Angiosarcoma
Clinical features
Angiosarcomas are biologically aggressive malignancies with high rates of mortality. More than half of patients die within a year of diagnosis (45). Most cases arise in the skin, followed by the deep soft tissues, breast, bone, and viscera. The mediastinum is a frequent site of involvement in children, who are only very rarely affected by angiosarcoma (18). Within the mediastinum, the anterior compartment is most often involved (50). The heart, great vessels, esophagus, and the thymus have all been documented as sites of origin (50-53). Angiosarcomas can arise secondarily within other tumors of the mediastinum, some examples including primary germ cell tumors (54,55) and schwannomas (56). Tumors are treated by resection, with or without chemotherapy (50,51,57). Some literature studies suggest a survival advantage for patients treated with the combination of surgery and radiation (58).
Pathologic features
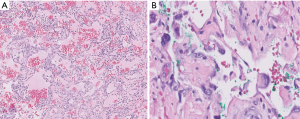
Tumor morphology can span a broad spectrum, ranging from strikingly low-grade appearances simulating hemangiomas to high-grade undifferentiated sarcomas (Figure 13). Vasoformation manifests as large cavernous spaces, irregular anastomosing channels, capillary-like proliferations, and slit-like channels lined by hyperchromatic endothelial cells. Vasoformation can be obscured when neoplastic cells grow in sheet-like, solid patterns. In areas of solid growth, tumor cells can be spindled or epithelioid. Some cases are entirely epithelioid (so-called epithelioid angiosarcoma). Essentially all cases exhibit hemorrhage, hemosiderin deposition, and multilayering of endothelial cells. Minor features include papillary projections resembling endothelial hyperplasia, pleomorphic tumor giant cells, fibrous or myxoid tumor stroma, and dissection into fat or other structures (50). Necrosis is often present, and mitotic activity appears to correlate with the degree of tumor differentiation (50). Foamy cell angiosarcoma is an extraordinarily rare and unusual variant of angiosarcoma which presents major diagnostic challenges and requires ancillary studies for diagnosis (Figure 14) (59).
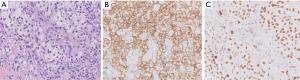
Ancillary studies
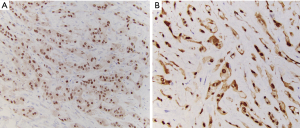
Angiosarcomas express both vascular and lymphatic markers (29). Expression of at least two vascular markers is recommended to underpin the diagnosis (Figure 14). Epithelioid cases exhibit some cytokeratin expression in about a quarter of cases, most commonly pankeratin, CK7, CAM5.2, or CK18 (37). MYC protein expression is most consistently observed among radiation-induced angiosarcomas of the breast, but there is limited utility of this marker at other anatomic sites (60). A positive result is more diagnostically useful than a negative one. MYC protein overexpression reflects MYC gene amplification, a consistent finding among post-irradiation angiosarcomas (less commonly FLT4 amplification, PTPBR mutation, and PLCH1 mutation). Primary angiosarcomas tend to exhibit complex karyotypes with occasional KDR or CIC mutations (22).
Differential diagnosis
Cases with epithelioid cytomorphology mimic carcinoma, an issue that can be further confounded by cytokeratin expression. Moreover, an epithelioid malignancy in the mediastinum is statistically far more likely to represent carcinoma than any mesenchymal neoplasm (61). Therefore, any evidence of vasoformation or a hemorrhagic background should trigger a panel of vascular markers. Distinction from mesothelioma would follow the same approach as discussed above for EHE. One potential pitfall is that D2-40 expression is seen in both mesothelioma and angiosarcoma. Spindled morphologic variants overlap with other sarcomas, but the typical immunohistochemical workup for sarcoma includes at least one vascular marker, which would indicate a vascular lineage. Spindle cell melanoma can exhibit pseudovascular spaces mimicking angiosarcoma (Figure 15), again requiring immunohistochemistry to nail down tumor origin. As mentioned above, some cases of EHE can pose a diagnostic dilemma, but the specialized matrix is the key to EHE. Molecular studies can be employed as an objective means to discriminate between the two.
Anastomosing hemangioma, which also occasionally occurs in the mediastinum, can simulate well-differentiated angiosarcoma (Figure 16) owing to its non-lobular, anastomosing growth pattern and mild nuclear atypia. Moreover, it may focally infiltrate into the surrounding soft tissue or viscera. However, anastomosing hemangioma lacks the diffusely infiltrative growth and other malignant features of angiosarcoma. Further diagnostic clues to anastomosing hemangioma include intralesional mature adipose tissue, small thrombi, and extramedullary hematopoiesis. The finding of a GNAQ mutation would also support a diagnosis of anastomosing hemangioma (62,63).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Saul Suster and David Suster) for the series “Mediastinal Sarcomas” published in Mediastinum. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-40). The series “Mediastinal Sarcomas” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Croteau SE, Gupta D. The clinical spectrum of kaposiform hemangioendothelioma and tufted angioma. Semin Cutan Med Surg 2016;35:147-52. [Crossref] [PubMed]
- Ji Y, Yang K, Peng S, et al. Kaposiform haemangioendothelioma: clinical features, complications and risk factors for Kasabach-Merritt phenomenon. Br J Dermatol 2018;179:457-63. [PubMed]
- Guleria P, Barwad A. Paediatric mesenchymal tumors of the mediastinum. Mediastinum 2020; [Crossref]
- O'Regan GM, Irvine AD, Yao N, et al. Mediastinal and neck kaposiform hemangioendothelioma: report of three cases. Pediatr Dermatol 2009;26:331-7. [Crossref] [PubMed]
- Wallenstein MB, Hole MK, McCarthy C, et al. Mediastinal kaposiform hemangioendothelioma and Kasabach-Merritt phenomenon in a patient with no skin changes and a normal chest CT. Pediatr Hematol Oncol 2014;31:563-7. [Crossref] [PubMed]
- Duan L, Renzi S, Weidman D, et al. Sirolimus Treatment of an Infant with Intrathoracic Kaposiform Hemangioendothelioma Complicated by Life-threatening Pleural and Pericardial Effusions. J Pediatr Hematol Oncol 2020;42:74-8. [Crossref] [PubMed]
- Beaton A, Kuttler T, Hassan A, et al. Hemangioendothelioma: a rare case of a primary intracardiac tumor. Pediatr Cardiol 2013;34:194-7. [Crossref] [PubMed]
- Drolet BA, Trenor CC 3rd, Brandão LR, et al. Consensus-derived practice standards plan for complicated Kaposiform hemangioendothelioma. J Pediatr 2013;163:285-91. [Crossref] [PubMed]
- Ji Y, Chen S, Yang K, et al. Kaposiform hemangioendothelioma: current knowledge and future perspectives. Orphanet J Rare Dis 2020;15:39. [Crossref] [PubMed]
- Putra J, Gupta A. Kaposiform haemangioendothelioma: a review with emphasis on histological differential diagnosis. Pathology 2017;49:356-62. [Crossref] [PubMed]
- Bhagalia SR, Pardhe N, Gupta M, et al. Juvenile hemangioma: A case report with an emphasis on its clinical phases (evolution and involution), and immunohistochemically distinctive physiologic differences. J Oral Maxillofac Pathol 2011;15:316-9. [Crossref] [PubMed]
- Ozeki M, Fujino A, Matsuoka K, et al. Clinical Features and Prognosis of Generalized Lymphatic Anomaly, Kaposiform Lymphangiomatosis, and Gorham-Stout Disease. Pediatr Blood Cancer 2016;63:832-8. [Crossref] [PubMed]
- Ozeki M, Fukao T. Generalized Lymphatic Anomaly and Gorham-Stout Disease: Overview and Recent Insights. Adv Wound Care (New Rochelle) 2019;8:230-45. [Crossref] [PubMed]
- Ji Y, Chen S, Peng S, et al. Kaposiform lymphangiomatosis and kaposiform hemangioendothelioma: similarities and differences. Orphanet J Rare Dis 2019;14:165. [Crossref] [PubMed]
- Liu Q, Ouyang R, Chen P, et al. A case report of retiform hemangioendothelioma as pleural nodules with literature review. Diagn Pathol 2015;10:194. [Crossref] [PubMed]
- Emberger M, Laimer M, Steiner H, et al. Retiform hemangioendothelioma: presentation of a case expressing D2-40. J Cutan Pathol 2009;36:987-90. [Crossref] [PubMed]
- Gambarotti M, Righi A, Sbaraglia M, et al. Intraosseous papillary intralymphatic angioendothelioma (PILA): one new case and review of the literature. Clin Sarcoma Res 2018;8:1. [Crossref] [PubMed]
- WHO Classification of Tumors Editorial Board. Soft Tissue and Bone Tumours. Lyon, France: IARC; 2020.
- Cakir E, Demirag F, Gulhan E, et al. Mediastinal composite hemangioendothelioma. A rare tumor at an unusual location. Tumori 2009;95:98-100. [Crossref] [PubMed]
- Fukunaga M, Suzuki K, Saegusa N, et al. Composite hemangioendothelioma: report of 5 cases including one with associated Maffucci syndrome. Am J Surg Pathol 2007;31:1567-72. [Crossref] [PubMed]
- Shang Leen SL, Fisher C, Thway K. Composite hemangioendothelioma: clinical and histologic features of an enigmatic entity. Adv Anat Pathol 2015;22:254-9. [Crossref] [PubMed]
- Papke DJ Jr, Hornick JL. What is new in endothelial neoplasia? Virchows Arch 2020;476:17-28. [Crossref] [PubMed]
- Pantanowitz L, Dezube BJ. Kaposi sarcoma in unusual locations. BMC Cancer 2008;8:190. [Crossref] [PubMed]
- Cipriano P, Nabais I, Melo N, et al. Chylous ascites in disseminated Kaposi sarcoma: an unusual manifestation as immune reconstitution inflammatory syndrome. BMJ Case Rep 2019;12:e228406 [Crossref] [PubMed]
- Schneider JW, Dittmer DP. Diagnosis and Treatment of Kaposi Sarcoma. Am J Clin Dermatol 2017;18:529-39. [Crossref] [PubMed]
- O'Donnell PJ, Pantanowitz L, Grayson W. Unique histologic variants of cutaneous Kaposi sarcoma. Am J Dermatopathol 2010;32:244-50. [PubMed]
- Chapalain M, Goldman-Lévy G, Kramkimel N, et al. Anaplastic Kaposi's sarcoma: 5 cases of a rare and aggressive type of Kaposi's sarcoma. Ann Dermatol Venereol 2018;145:21-8. [Crossref] [PubMed]
- Yu Y, Demierre MF, Mahalingam M. Anaplastic Kaposi's sarcoma: an uncommon histologic phenotype with an aggressive clinical course. J Cutan Pathol 2010;37:1088-91. [Crossref] [PubMed]
- Breiteneder-Geleff S, Soleiman A, Kowalski H, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol 1999;154:385-94. [Crossref] [PubMed]
- Ueda K. KSHV Genome Replication and Maintenance in Latency. Adv Exp Med Biol 2018;1045:299-320. [Crossref] [PubMed]
- McGinity M, Bartanusz V, Dengler B, et al. Pseudomyogenic hemangioendothelioma (epithelioid sarcoma-like hemangioendothelioma, fibroma-like variant of epithelioid sarcoma) of the thoracic spine. Eur Spine J 2013;22:S506-S511. [Crossref] [PubMed]
- Hornick JL, Fletcher CD. Pseudomyogenic hemangioendothelioma: a distinctive, often multicentric tumor with indolent behavior. Am J Surg Pathol 2011;35:190-201. [Crossref] [PubMed]
- Pradhan D, Schoedel K, McGough RL, et al. Pseudomyogenic hemangioendothelioma of skin, bone and soft tissue-a clinicopathological, immunohistochemical, and fluorescence in situ hybridization study. Hum Pathol 2018;71:126-34. [Crossref] [PubMed]
- Hung YP, Fletcher CD, Hornick JL. FOSB is a Useful Diagnostic Marker for Pseudomyogenic Hemangioendothelioma. Am J Surg Pathol 2017;41:596-606. [Crossref] [PubMed]
- Sugita S, Hirano H, Kikuchi N, et al. Diagnostic utility of FOSB immunohistochemistry in pseudomyogenic hemangioendothelioma and its histological mimics. Diagn Pathol 2016;11:75. [Crossref] [PubMed]
- Agaram NP, Zhang L, Cotzia P, et al. Expanding the Spectrum of Genetic Alterations in Pseudomyogenic Hemangioendothelioma With Recurrent Novel ACTB-FOSB Gene Fusions. Am J Surg Pathol 2018;42:1653-61. [Crossref] [PubMed]
- Anderson T, Zhang L, Hameed M, et al. Thoracic epithelioid malignant vascular tumors: a clinicopathologic study of 52 cases with emphasis on pathologic grading and molecular studies of WWTR1-CAMTA1 fusions. Am J Surg Pathol 2015;39:132-9. [Crossref] [PubMed]
- Suster S, Moran CA, Koss MN. Epithelioid hemangioendothelioma of the anterior mediastinum. Clinicopathologic, immunohistochemical, and ultrastructural analysis of 12 cases. Am J Surg Pathol 1994;18:871-81. [Crossref] [PubMed]
- den Bakker MA, Marx A, Mukai K, et al. Mesenchymal tumours of the mediastinum--part II. Virchows Arch 2015;467:501-17. [Crossref] [PubMed]
- Rosenbaum E, Jadeja B, Xu B, et al. Prognostic stratification of clinical and molecular epithelioid hemangioendothelioma subsets. Mod Pathol 2020;33:591-602. [Crossref] [PubMed]
- Deyrup AT, Tighiouart M, Montag AG, et al. Epithelioid hemangioendothelioma of soft tissue: a proposal for risk stratification based on 49 cases. Am J Surg Pathol 2008;32:924-7. [Crossref] [PubMed]
- Lamar JM, Motilal Nehru V, Weinberg G. Epithelioid Hemangioendothelioma as a Model of YAP/TAZ-Driven Cancer: Insights from a Rare Fusion Sarcoma. Cancers (Basel) 2018;10:229. [Crossref] [PubMed]
- Suurmeijer AJH, Dickson BC, Swanson D, et al. Variant WWTR1 gene fusions in epithelioid hemangioendothelioma-A genetic subset associated with cardiac involvement. Genes Chromosomes Cancer 2020;59:389-95. [Crossref] [PubMed]
- Antonescu CR, Le Loarer F, Mosquera JM, et al. Novel YAP1-TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes Chromosomes Cancer 2013;52:775-84. [Crossref] [PubMed]
- Antonescu C. Malignant vascular tumors--an update. Mod Pathol 2014;27:S30-S38. [Crossref] [PubMed]
- Shibuya R, Matsuyama A, Shiba E, et al. CAMTA1 is a useful immunohistochemical marker for diagnosing epithelioid haemangioendothelioma. Histopathology 2015;67:827-35. [Crossref] [PubMed]
- Lin BT, Colby T, Gown AM, et al. Malignant vascular tumors of the serous membranes mimicking mesothelioma. A report of 14 cases. Am J Surg Pathol 1996;20:1431-9. [Crossref] [PubMed]
- Zhang PJ, Livolsi VA, Brooks JJ. Malignant epithelioid vascular tumors of the pleura: report of a series and literature review. Hum Pathol 2000;31:29-34. [Crossref] [PubMed]
- Luzar B, Ieremia E, Antonescu CR, et al. Cutaneous intravascular epithelioid hemangioma. A clinicopathological and molecular study of 21 cases. Mod Pathol 2020; [Epublished ahead of print]. [Crossref] [PubMed]
- Weissferdt A, Kalhor N, Suster S, et al. Primary angiosarcomas of the anterior mediastinum: a clinicopathologic and immunohistochemical study of 9 cases. Hum Pathol 2010;41:1711-7. [Crossref] [PubMed]
- Fatima J, Duncan AA, Maleszewski JJ, et al. Primary angiosarcoma of the aorta, great vessels, and the heart. J Vasc Surg 2013;57:756-64. [Crossref] [PubMed]
- Wu J, Li X, Liu X. Epithelioid angiosarcoma: a clinicopathological study of 16 Chinese cases. Int J Clin Exp Pathol 2015;8:3901-9. [PubMed]
- Chan Y, El-Zimaity H, Darling GE. Primary angiosarcoma of the esophagus. Ann Thorac Surg 2013;95:e19-e20. [Crossref] [PubMed]
- Contreras AL, Punar M, Tamboli P, et al. Mediastinal germ cell tumors with an angiosarcomatous component: a report of 12 cases. Hum Pathol 2010;41:832-7. [Crossref] [PubMed]
- Matsuoka S, Koyama T, Takeda T, et al. Development of angiosarcoma in a mediastinal non-seminomatous germ cell tumor that exhibited growing teratoma syndrome during chemotherapy. Thorac Cancer 2019;10:111-5. [Crossref] [PubMed]
- Demiröz ŞM, Fındık G, Aydoğdu K, et al. Mediastinal epithelioid angiosarcoma arising in schwannoma: The first case in the literature. Turk Gogus Kalp Damar Cerrahisi Derg 2018;26:305-8. [Crossref] [PubMed]
- Tane S, Tanaka Y, Tauchi S, et al. Radically resected epithelioid angiosarcoma that originated in the mediastinum. Gen Thorac Cardiovasc Surg 2011;59:503-6. [Crossref] [PubMed]
- Engelhardt KE, DeCamp MM, Yang AD, et al. Treatment Approaches and Outcomes for Primary Mediastinal Sarcoma: Analysis of 976 Patients. Ann Thorac Surg 2018;106:333-9. [Crossref] [PubMed]
- Svajdler M, Benický M, Fröhlichová L, et al. Foamy cell angiosarcoma is a diagnostic pitfall: a case report of an angiosarcoma mimicking xanthoma. Am J Dermatopathol 2014;36:669-72. [PubMed]
- Ginter PS, Mosquera JM, MacDonald TY, et al. Diagnostic utility of MYC amplification and anti-MYC immunohistochemistry in atypical vascular lesions, primary or radiation-induced mammary angiosarcomas, and primary angiosarcomas of other sites. Hum Pathol 2014;45:709-16. [Crossref] [PubMed]
- den Bakker MA, Marx A, Mukai K, et al. Mesenchymal tumours of the mediastinum--part I. Virchows Arch 2015;467:487-500. [Crossref] [PubMed]
- Bean GR, Joseph NM, Gill RM, et al. Recurrent GNAQ mutations in anastomosing hemangiomas. Mod Pathol 2017;30:722-7. [Crossref] [PubMed]
- Bean GR, Joseph NM, Folpe AL, et al. Recurrent GNA14 mutations in anastomosing haemangiomas. Histopathology 2018;73:354-7. [Crossref] [PubMed]
Cite this article as: Paral K, Krausz T. Vascular tumors of the mediastinum. Mediastinum 2020;4:25.