
The role of EBUS-TBNA in lung cancer restaging and mutation analysis
Introduction
Despite the advances of multimodality treatments, lung cancer is still one of the world-leading causes of death. It has been estimated that over 228,000 people will be diagnosed with lung cancer in 2020 in the USA, and up to 135,000 individuals will die of the disease (1). According to European data (2), pulmonary tumors represent the first cause of death for neoplasm in the male sex, and the second in women.
Non-small cell lung cancer (NSCLC) accounts for 85% of all cases (3) and up to 65% of the patients have locally advanced or metastatic disease at the time of diagnosis (4,5).
In recent years, molecular targeted treatments have progressively entered in standard therapeutic regimens for stage III–IV NSCLC (6,7). The latest update of NCCN guidelines (8) recommends osimertinib as first-line treatment in patients with positive EGFR mutation, and alectinib in those with ALK rearrangement. Both NCCN and ASCO (9) guidelines strongly recommend the use of pembrolizumab, an immune checkpoint PD-1 inhibitor (ICI), as first-line treatment of patients with high PD-L1 expression (>50%), alone or in association with platinum-based regimens.
Molecular-based treatments are, however, not only reserved to patients with advanced, unresectable disease. There is indeed growing evidence that even subjects with limited disease may benefit from targeted therapies: several trials are investigating the use of tyrosine kinase inhibitors (TKIs) and ICIs as alternative neoadjuvant and adjuvant options for early stage NSCLC, or in case of recurrence after complete treatment (10,11).
At the present time, seven EGFR inhibitors, five drugs targeting ALK changes, four targeting abnormal ROS1, 2 for BRAF gene changes, two interfering with NTRK gene changes, and four PD-1/PD-L1 blocking agents are available, and some of them were approved for the treatment of mutation-bearing NSCLC in the clinical practice (12,13). This is the reason why the search of a broad molecular analysis, including targetable gene aberrations and immunohistochemistry for PD-L1 testing, is strongly recommended at the time of diagnosis of NSCLC, not only on surgical specimens but also on small biopsy samples (8,14).
Several guidelines suggest EBUS-TBNA as the procedure of choice for the diagnosis and staging of patients with suspected NSCLC. The American College of Chest Physicians (ACCP) guidelines for the diagnosis and staging of lung cancer (15) recommend EBUS-TBNA both in case of enlarged lymph nodes regardless of PET uptake, and in patients with normal-size lymph nodes at CT scan showing pathologic FDG uptake at whole body-PET scan. Moreover, ACCP guidelines and the combined guidelines of the European Society of Gastrointestinal Endoscopy (ESGE), the European Respiratory Society (ERS), and the European Society of Thoracic Surgeons (ESTS) (16) both advise that patients undergoing pulmonary resection for NSCLC should be preoperatively staged with EBUS-TBNA in case of tumors larger than 3 cm, centrally located lesions, or PET negative primary tumors.
In a recent prospective multicentric study (17), EBUS-TBNA showed a higher diagnostic yield when compared to any other bronchoscopic sampling technique and resulted to be independently associated with a higher probability of diagnosis at multivariate analysis. The combination (CUS) of EBUS-TBNA and endoscopic ultrasound-fine needle aspiration (EUS-FNA) allows complete staging of the mediastinum in patients with NSCLC, reaching a sensitivity value even superior to that of cervical mediastinoscopy (18), and should therefore be preferred whenever available (15,16).
With the discovery of the therapeutic value of targetable EGFR mutation in 2004, the availability of an adequate amount of tissue for histology subtyping and molecular analysis became a critical issue. Despite the uncertain consistency of the initial results (19), small samples and even cytological specimens proved to be appropriate for a full molecular assessment of NSCLC when properly handled (20,21). Moreover, in the studies by Heymann et al. (22) and Verocq et al. (23), the immunohistochemical analysis of PD-L1 expression on cytological and small biopsy samples from patients affected by NSCLC resulted comparable to the corresponding surgical samples.
Nowadays, EBUS-TBNA is a key diagnostic tool in patients with locally advanced or unresectable disease and for patients unfit for surgery because of comorbidities, reducing the need of invasive surgical diagnostic procedures. Considering these premises, EBUS-TBNA not only plays a key role in the diagnosis and staging of suspected lung cancer, but it also proved to allow accurate molecular characterization of the disease.
Adequacy of molecular genotyping and PD-L1 assessment on samples obtained by EBUS-TBNA: review of literature
In 2007, Nakajima et al. (24) first assessed the feasibility of EGFR mutation determination on samples obtained by EBUS-TBNA. In 43 out of 46 patients (93.5%) with newly diagnosed locally advanced or metastatic lung adenocarcinoma enrolled in the study, analysis of exons 19 and 21 of EGFR gene was possible after polymerase chain reaction (PCR) on histological core-biopsy tissue. The Authors concluded that EBUS-TBNA was an appropriate technique for EGFR mutation analysis; notably, specimens had a lower burden of contaminating cells with respect to those obtained with other non-surgical sampling techniques.
So far, a number of other studies investigated the adequacy of EBUS-TBNA samples for the search of several biomarkers (Table 1). Gefinitib and crizotinib were the first TKIs approved for the treatment of metastatic lung cancer patients, respectively expressing EGFR mutation and ALK translocation. Considering the higher rates of positive samples found in female, non-smoker patients with adenocarcinoma histology (51), molecular assessment was at first almost exclusively reserved to these cases. Moreover, as incidence of EGFR alterations is relatively higher in Asian race compared to Caucasians (ranging from 14% to 27%), Japanese groups were the first to report their experience on the topic (26,27,30).
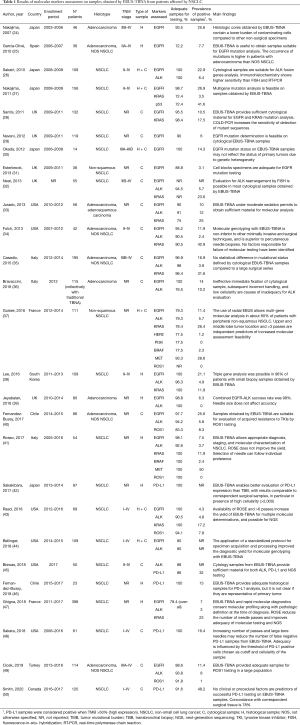
Full table
Most Authors agree that the diagnostic yield of EBUS-TBNA for molecular genotyping is high. In some studies, EGFR and ALK determination was possible in the entire cohort of the patients enrolled (26,30,36,38,39,43), and in most of the experiences adequate specimens were available in over 90% of patients who underwent EBUS-TBNA. In 2018, Labarca et al. released a meta-analysis including 33 studies (almost 2,700 patients) evaluating the diagnostic power of EBUS-TBNA for NSCLC molecular characterization (52). The pooled diagnostic yield for EGFR and ALK determination reached 94.5% and 94.9%, respectively; combined EGFR and ALK analysis, reported by 9 of the trials analyzed, was successful in 94.2% of cases.
As a result of the introduction of new molecules, improved diagnostic and therapeutic pathway of lung cancer, and increased confidence with the technique, indication for molecular assessment on EBUS-TBNA samples has now been extended to patients with histotypes different from adenocarcinoma, as well as to those with limited disease. Guisier and colleagues (37) investigated the presence of multiple gene aberrations (including EGFR, ALK, KRAS, MET, and ROS1) in 111 patients with peripheral non-squamous NSCLC who underwent sampling with radial EBUS-TBNA. Biopsy tissue resulted adequate in about 80% of cases. Other trials confirmed the possibility to perform multiple molecular analyses on EBUS-TBNA samples, some reporting a percentage of sample adequacy even superior to 90% (38,41,43).
Patients showing with locally advanced or metastatic NSCLC, with wild-type EGFR and ALK and PD-L1 expression in over 50% of neoplastic cell population [i.e., tumor mutational burden (TMB)] at immunochemistry (IHC) are suitable for the treatment with PD-1 or PD-L1 ICIs (Figure 1). Significant results in terms of both local disease control and improvement of survival were demonstrated following treatment with these molecules (13). Considering that most patients with stage III–IV disease do not undergo surgical procedures, collection of adequate samples for IHC analysis by EBUS-TBNA has gained a prominent role.
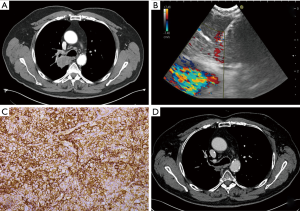
Only few studies analyzed the feasibility of performance of PD-L1 testing by means of EBUS-TBNA, with diagnostic yield ranging from 86% and 100% of the patients tested (42,45,46,48,50). In the series by Sakakibara et al. (42), EBUS-TBNA samples showed a higher cellularity and contained better conserved tumoral cells with respect to those obtained with conventional transbronchial biopsy (TBB). Moreover, results of PD-L1 assessment were concordant to primary tumors and lymph node metastases with a good rate of correlation, as confirmed by another study (50).
As in case of other molecular biomarkers, cytological specimens demonstrated to be appropriate for a full analysis of PD-L1 with the currently available IHC platforms in the presence of adequate cellularity (45,48,50). Additional passes and large bore needles have been suggested to reduce confounding results due to possible tumor heterogeneity and choice of PD-L1 threshold; nevertheless, Smith and colleagues did not identify any significant procedural influencing factor (50).
Technical factors affecting accuracy of mutation analysis on EBUS-TBNA samples
It was demonstrated that accuracy of molecular analysis on lung cancer samples obtained by EBUS-TBNA is influenced by several intrinsic factors related to the tumor characteristics, such as histologic subtype, tumor location, target lymph node size, and grade of tumor heterogeneity between primary lesion and metastatic sites (30,37,42,46,53). Moreover, other factors potentially conditioning the rate of success are mutation prevalence in the examined population and ethnicity (52).
The role of technical features involved in EBUS-TBNA outcome for the search of molecular aberrations has been widely investigated. Several studies pointed out that the choice of needle, number of passes, use of rapid-on-site-evaluation (ROSE), sample cellularity and contamination by surrounding necrosis or blood elements, and sample processing are determinant factors to obtain suitable material (33). The CHEST guidelines for EBUS-TBNA released in 2016 recommend, regardless of ROSE availability, at least three passes for each sampled station, and possibly additional passes to increase effectiveness of mutation analysis, but with low level of evidence (54).
Others did not confirm these findings with contrasting results (34). The meta-analysis of Labarca et al. (52) failed to identify any procedural feature significantly correlated to provision of adequate material for molecular investigations.
Needle size and type of sample
In most of the published series, cytological and histological samples are obtained with the employment of 21- or 22-gauge needles. Although Authors supporting the use of larger bore needles confirm a similar diagnostic performance to 22-gauge needle, they report that samples obtained employing 21-gauge needles display conserved architecture, allowing better morphologic and genetic characterization of NSCLC (55). On the other side, 22-gauge needle has the advantage of being able to reach ‘difficult’ locations, such as 4L lymph node station, thanks to its flexibility.
Jeyabalan et al. (39) and Rosso and colleagues (41) analyzed the potential effect produced by the choice of needles of different size; both Authors, however, concluded that, given the comparable results, selection should follow the individual preference of the operator, as suggested by CHEST guidelines (54).
Number of passes and ROSE
In 2013, Yarmus and colleagues (56) analyzed the data of 85 patients affected by lung adenocarcinoma or not otherwise specified (NOS) NSCLC. Excellent results for mutation analysis including EGFR, ALK, and KRAS were obtained in patients submitted to at least 4 passes per sampled site and concurrent ROSE. Raad et al. stated that the rate of success could be increased by carrying out more than 6 passes in a Center with ROSE availability (43). In some cases, even higher number of biopsies (up to 20) have been reported (42).
The studies addressing the use of ROSE in patients undergoing EBUS-TBNA for genotyping of NSCLC gave discordant results. According to Ghigna et al. (47), fresh-frozen samples sent for on-site examination provide uncrushed genetic material for ancillary tests of higher quality than fixed samples. A randomized trial comparing two groups of NSCLC patients who underwent molecular analysis with or without ROSE found no significant difference in terms of sensitivity and adequacy rate (57). Further investigations confirmed that, if an adequate number of passes per sampled station is performed (usually 3 to 4), it is possible to obtain a full molecular diagnosis of NSCLC regardless of the availability of ROSE (37,41,48,53). Hence, the use of ROSE is not mandatory, but it should be tailored on the basis of Center experience (54).
In the daily clinical practice, however, shortage of material for mutation analysis after routine processing for cytological and IHC analysis is common. Nevertheless, it has been showed that material obtained by even a single dedicated additional pass may provide sufficient material for a full molecular assessment (58), a factor that should always be considered to ensure adequate diagnosis, staging and molecular characterization of suspect lung cancer.
Sample management and detection method
Regardless of needle size, EBUS-TBNA sampled material can be processed in several ways both for histological and/or cytological examination. Cytological specimens may be smeared on glass slides or assembled as paraffin-embedded cell blocks following individual preferences.
In 2010, the conjunct consensus released by the International Association for the Study of Lung Cancer (IASLC) and the European Thoracic Oncology Platform (59) advised the use of core biopsies for EGFR characterization until better definition of the role of cytological specimens; similar conclusions are reported by IASLC for IHC analysis of PD-L1 (60).
Nevertheless, several studies analyzing the results of molecular determination and PD-L1 determination on EBUS-TBNA samples so far demonstrated that cytological specimens, in particular cell blocks, enable high quality processing for such purposes (26,28,29,31,32,35,45). Bravaccini et al. (36) reported that wrong specimen handling after withdrawal rather than the amount of tissue available for analysis is responsible for missing diagnosis and molecular characterization.
According to the guidelines of the World Association for Bronchology and Interventional Pulmonology (WABIP), none between smear glass cytology, cell block and tissue core biopsy is superior to the others to improve the likelihood to obtain adequate samples (61). In fact, cellularity of the sample, ratio between normal and tumoral cells, and performance of the adopted method of detection seem to be the factors mostly influencing the diagnostic yield. In most of the published series, PCR is the preferred method for amplification of target sequences; yet, the quantity of tumoral DNA necessary for completion of analysis may vary according to the used technique (28). With regard to ALK analysis, there are some evidences supporting superiority of IHC over fluorescence-in-situ- hybridization (FISH) and real-time PCR (RT-PCR) (26). However, no detection method demonstrated to be superior to others in the meta-analysis of Labarca et al. (52).
Lung cancer restaging and EBUS-TBNA
Stage III of tumor-node-metastasis (TNM) staging system for NSCLC includes a variety of clinical presentations ranging from large pulmonary masses invading neighboring structures to small primary lesions with mediastinal lymph node metastases. Despite upfront surgery may be an option in carefully selected patients (e.g., in case of single N2 station disease) (62), it is widely accepted that primary resection without a preliminary induction therapy is detrimental because of high risk of incomplete resection and later recurrence (63).
In recent years, 18-F-FDG-PET scan demonstrated to be a useful tool to ensure appropriate staging of both primary tumor and regional and distant metastases (8). Still, some questions were raised regarding the efficiency of imaging for disease restaging after induction treatments. A recent systematic review underlined that, even if SUVmax and other newly introduced metabolic parameters seem to be promising factors for the evaluation of response, further larger trials are required to confirm the results (64).
Therefore, pathologic assessment after neoadjuvant therapy should still be considered mandatory for an appropriate therapeutic planning. Repeated mediastinoscopy or more invasive surgical approaches have been for a long time the only available techniques for preoperative evaluation of patients undergoing radical treatment. However, many Authors reported non-negligible rates of morbidity and mortality, and inadequate sensitivity and accuracy as consequences of technical challenges caused by the presence of inflammatory fibrosis induced by primary staging mediastinoscopy and oncological treatment (65).
The advent of EBUS-TBNA offered the possibility of a minimally invasive staging, and an improvement of the results of imaging staging when used in association with PET-CT scan (66). A summary of studies that investigated the role of EBUS-TBNA in mediastinal restaging is reported in Table 2.
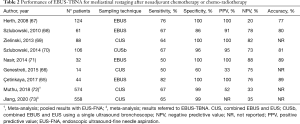
Full table
Mediastinal restaging with EBUS-TBNA after induction chemotherapy was first assessed in 2008 in a trial including 89 patients (67). Samples resulted positive in all patients showing stable disease at restaging CT scan and persistent metastases at subsequent intraoperative biopsy. However, 28 out of 35 EBUS-negative patients were found to have lymph node metastases at the time of surgery, resulting in suboptimal sensitivity and low (20%) negative predictive value (NPV). Nevertheless, the authors pointed out that these results were comparable to those obtained in patients who underwent induction therapy on the basis of non-invasive primary staging and later restaged with mediastinoscopy, and superior to repeated mediastinoscopy.
Several studies remarked the presence of a fair number of false negative patients influencing the NPV of EBUS for mediastinal restaging. Probably, residual cancer cells may be not detected in small EBUS samples, because of necrosis and fibrosis induced by neoadjuvant chemo- and radiotherapy (74). Nevertheless, some authors reported values of NPV superior to 80%, not as high as those obtained by surgical restaging, but with a significant lower rate of procedural morbidity (69,71).
The association of EBUS-TBNA and EUS-FNA may improve the performance of ultrasonographic restaging (69). Szlubowski and colleagues (70) showed that a full mediastinal restaging with combined EBUS and EUS is feasible with a single scope instrument (CUSb-NA). Both sensitivity and accuracy of CUSb-NA resulted significantly higher than EBUS-TBNA and EUS-FNA alone. However, these results have not been confirmed in another study with a smaller population (66).
Guidelines for the selection of endoscopic or surgical restaging of NSCLC are still lacking. However, considering the number of false negative patients found at restaging with EBUS-TBNA, pathologic surgical confirmation [either with mediastinoscopy, transcervical extended mediastinal lymphadenectomy (TEMLA), VATS, or thoracotomy] seems to be still advisable before considering definitive treatment, as confirmed by two recent meta-analyses (72,73).
Some patients treated with targeted therapy with TKIs or ICIs show incomplete response or tumor progression at follow up. In fact, the onset of new gene mutations inducing resistance to first-line treatments and tumor transformation into more aggressive, less differentiated histologic subtypes are well known phenomena (75-77). This is why disease restaging could help in the definition of the following treatment. Recovery times being notably shorter, restaging by means of EBUS-TBNA may reduce the time interval between diagnosis and therapy onset compared to surgical procedures. However, there are still few reports investigating the role of EBUS-TBNA for molecular restaging.
Kirita et al. (78) analyzed 70 patients with NSCLC who developed resistance to standard chemotherapy or targeted drugs. Eighteen patients were rebiopsied by means of EBUS-TBNA, and 52 with TBB. All EBUS-TBNA cases resulted diagnostic, compared to 83% of TBBs, even if the difference was not statistically significant. Genotyping was possible in all cases; one patient showed small-cell lung cancer (SCLC) transformation.
In another study by Izumo et al. (79), molecular analysis was required in 53 NSCLC patients previously treated with TKIs who developed resistance. The rate of adequate samples for mutation analysis was higher in the group of patients who underwent EBUS-TBNA compared to TBB under EBUS guidance (100% vs. 75%, respectively). In both Kirita and Izumo series no complications related to EBUS-TBNA procedure have been reported, confirming the safety of the technique. However, the number of patients enrolled in these trials was low, and larger studies are required to confirm these results.
Next-generation sequencing (NGS) and future perspectives
In most of the studies investigating the feasibility of molecular analysis on samples obtained by EBUS-TBNA, only one or two genes were tested for target aberrations search (Table 1). Some Authors demonstrated that both cytological and histological EBUS-TBNA specimens are suitable for multiple analyses (37,41,43). However, wasting of material is still a problem to be faced, and accurate selection of molecular tests is an essential step to achieve adequate characterization of the disease. Nevertheless, the number of available therapeutic molecules for mutated NSCLC is rapidly increasing (12), as well as the alterations to be analyzed on the available tissue.
NGS is a novel technique that enables the simultaneous identification of a large panel (from 50 to over 1,000) of gene alterations—including target driver mutations—assessed on a single platform (80). Hence, NGS is going to cover an important role in the therapeutic decision for patients undergoing targeted therapies, immunotherapy, and enrollment in clinical trials.
A few studies investigated the feasibility of NGS on samples obtained by EBUS-TBNA. One of the main limitations for its application is due to the necessity of increasing gradients of cellularity in the specimen according to the number of genes that have to be assessed. For this reason, the adequacy of small EBUS-TBNA samples for such purpose has been a note of concern.
Yet, it has been demonstrated that NGS analysis can be carried out not only on tissue core biopsies, but also on cell blocks, and even on cytology smears (81,82). Several studies reported a successful analysis in over 90% of EBUS-TBNA samples submitted for NGS (83-85).
In experienced centers, EBUS-TBNA has therefore emerged as a technique that enables provision of adequate specimens for a full molecular assessment in patients affected by NSCLC. Future research in the field of molecular analysis with EBUS-TBNA should be directed to the standardization of the sampling technique and tissue management, and the development of dedicated guidelines.
The low NPV, in particular in case of NSCLC restaging, is one of the main limitations of EBUS-TBNA, and studies are being carried out to overcome the high number of false negative cases with new more specific detection targets. Inage and colleagues (86) investigated the role of microRNAs assessment as tumor markers in patients undergoing NSCLC restaging after chemo-radiotherapy with encouraging results, as they were able to reach a NPV of 100%. However, further trials are needed to definitely improve the effectiveness of EBUS-TBNA in this field.
Conclusions
Samples obtained by EBUS-TBNA from patients affected by NSCLC are adequate for a full genetic profiling of alterations that can be targeted by tailored treatments. Despite the high number of technical variables involved (type of needle, number of passes, use of ROSE, sample management, detection method), none of these factors seems to sensitively affect the overall diagnostic yield of the technique.
The use of EBUS-TBNA should be encouraged in patients with NSCLC who need a restaging of disease after induction therapy, or progression in the course of therapy with TKIs or ICIs, to guide subsequent treatments. However, considering the relatively high number of false negative cases, it is still advisable to offer a surgical biopsy to patients without evidence of tumor cells on EBUS-TBNA specimen before definitive treatment.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Angelo Carretta) for the series “Minimally Invasive (Endoscopic) Mediastinal Staging of Lung Cancer” published in Mediastinum. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-24). The series “Minimally Invasive (Endoscopic) Mediastinal Staging of Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 2018;103:356-87. [Crossref] [PubMed]
- Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc 2019;94:1623-40. [Crossref] [PubMed]
- Morgensztern D, Ng SH, Gao F, et al. Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J Thorac Oncol 2010;5:29-33. [Crossref] [PubMed]
- Lin J, McGlynn KA, Nations JA, et al. Comorbidity and stage at diagnosis among lung cancer patients in the US military health system. Cancer Causes Control 2020;31:255-61. [Crossref] [PubMed]
- Tsao AS, Jolly S, Lee JM. Updates in local-regionally advanced non-small cell lung cancer. Am Soc Clin Oncol Educ Book 2019;39:553-62. [Crossref] [PubMed]
- Puri S, Saltos A, Perez B, et al. Locally advanced, unresectable non-small cell lung cancer. Curr Oncol Rep 2020;22:31. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Non-small cell lung cancer (version 3.2020). [Accessed 27th March 2020]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Hanna NH, Schneider BJ, Temin S, et al. Therapy for stage IV non-small-cell lung cancer without driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol 2020;38:1608-32. [Crossref] [PubMed]
- Sandler JE, D'Aiello A, Halmos B. Changes in store for early-stage non-small cell lung cancer. J Thorac Dis 2019;11:2117-25. [Crossref] [PubMed]
- Vansteenkiste J, Wauters E, Reymen B, et al. Current status of immune checkpoint inhibition in early-stage NSCLC. Ann Oncol 2019;30:1244-53. [Crossref] [PubMed]
- American Cancer Society. Targeted therapy for non-small cell lung cancer. [Accessed 9th April 2020]. Available online: https://www.cancer.org/cancer/lung-cancer/treating-non-small-cell/targeted-therapies.html
- Lim SM, Hong MH, Kim HR. Immunotherapy for non-small cell lung cancer: current landscape and future perspectives. Immune Netw 2020;20:e10 [Crossref] [PubMed]
- Kalemkerian GP, Narula N, Kennedy EB, et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J Clin Oncol 2018;36:911-19. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Vilmann P, Frost Clementsen P, Colella S, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2015;48:1-15. [Crossref] [PubMed]
- Silvestri GA, Bevill BT, Huang J, et al. An evaluation of diagnostic yield from bronchoscopy: the impact of clinical/radiographic factors, procedure type, and degree of suspicion for cancer. Chest 2020;157:1656-64. [Crossref] [PubMed]
- McLean AEB, Barnes DJ, Troy LK. Diagnosing lung cancer: the complexities of obtaining a tissue diagnosis in the era of minimally invasive and personalised medicine. J Clin Med 2018;7:163. [Crossref] [PubMed]
- Bubendorf L, Lantuejoul S, de Langen AJ, et al. Nonsmall cell lung carcinoma: diagnostic difficulties in small biopsies and cytological specimens. Eur Respir Rev 2017;26:170007 [Crossref] [PubMed]
- Lozano MD, Echeveste JI, Abengozar M, et al. Cytology smears in the era of molecular biomarkers in non-small cell lung cancer: doing more with less. Arch Pathol Lab Med 2018;142:291-8. [Crossref] [PubMed]
- Roh MH. The utilization of cytologic and small biopsy samples for ancillary molecular testing. Mod Pathol 2019;32:77-85. [Crossref] [PubMed]
- Heymann JJ, Bulman WA, Swinarski D, et al. PD-L1 expression in non-small cell lung carcinoma: Comparison among cytology, small biopsy, and surgical resection specimens. Cancer Cytopathol 2017;125:896-907. [Crossref] [PubMed]
- Verocq C, Decaestecker C, Rocq L, et al. The daily practice reality of PD-L1 (CD274) evaluation in non-small cell lung cancer: a retrospective study. Oncol Lett 2020;19:3400-10. [PubMed]
- Nakajima T, Yasufuku K, Suzuki M, et al. Assessment of epidermal growth factor receptor mutation by endobronchial ultrasound-guided transbronchial needle aspiration. Chest 2007;132:597-602. [Crossref] [PubMed]
- Garcia-Olivé I, Monsó E, Andreo F, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for identifying EGFR mutations. Eur Respir J 2010;35:391-5. [Crossref] [PubMed]
- Sakairi Y, Nakajima T, Yasufuku K, et al. EML4-ALK fusion gene assessment using metastatic lymph node samples obtained by endobronchial ultrasound-guided transbronchial needle aspiration. Clin Cancer Res 2010;16:4938-45. [Crossref] [PubMed]
- Nakajima T, Yasufuku K, Nakagawara A, et al. Multigene mutation analysis of metastatic lymph nodes in non-small cell lung cancer diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration. Chest 2011;140:1319-24. [Crossref] [PubMed]
- Santis G, Angell R, Nickless G, et al. Screening for EGFR and KRAS mutations in endobronchial ultrasound derived transbronchial needle aspirates in non-small cell lung cancer using COLD-PCR. PLoS One 2011;6:e25191 [Crossref] [PubMed]
- Navani N, Brown JM, Nankivell M, et al. Suitability of endobronchial ultrasound-guided transbronchial needle aspiration specimens for subtyping and genotyping of non-small cell lung cancer: a multicenter study of 774 patients. Am J Respir Crit Care Med 2012;185:1316-22. [Crossref] [PubMed]
- Okada H, Anayama T, Kume M, et al. Comparison of epidermal growth factor receptor mutation analysis results between surgically resected primary lung cancer and metastatic lymph nodes obtained by endobronchial ultrasound-guided transbronchial needle aspiration. Thorac Cancer 2012;3:262-8. [Crossref] [PubMed]
- Esterbrook G, Anathhanam S, Plant PK. Adequacy of endobronchial ultrasound transbronchial needle aspiration samples in the subtyping of non-small cell lung cancer. Lung Cancer 2013;80:30-4. [Crossref] [PubMed]
- Neat MJ, Foot NJ, Hicks A, et al. ALK rearrangements in EBUS-derived transbronchial needle aspiration cytology in lung cancer. Cytopathology 2013;24:356-64. [Crossref] [PubMed]
- Jurado J, Saqi A, Maxfield R, et al. The efficacy of EBUS-guided transbronchial needle aspiration for molecular testing in lung adenocarcinoma. Ann Thorac Surg 2013;96:1196-202. [Crossref] [PubMed]
- Folch E, Yamaguchi N, VanderLaan PA, et al. Adequacy of lymph node transbronchial needle aspirates using convex probe endobronchial ultrasound for multiple tumor genotyping techniques in non-small-cell lung cancer. J Thorac Oncol 2013;8:1438-44. [Crossref] [PubMed]
- Casadio C, Guarize J, Donghi S, et al. Molecular testing for targeted therapy in advanced non-small cell lung cancer: suitability of endobronchial ultrasound transbronchial needle aspiration. Am J Clin Pathol 2015;144:629-34. [Crossref] [PubMed]
- Bravaccini S, Tumedei MM, Ulivi P, et al. ALK translocation detection in non-small cell lung cancer cytological samples obtained by TBNA or EBUS-TBNA. Cytopathology 2016;27:103-7. [Crossref] [PubMed]
- Guisier F, Salaün M, Lachkar S, et al. Molecular analysis of peripheral non-squamous non-small cell lung cancer sampled by radial EBUS. Respirology 2016;21:718-26. [Crossref] [PubMed]
- Lee K, Um SW, Jeong BH, et al. Triple gene analysis using samples obtained by endobronchial ultrasound-guided transbronchial needle aspiration. Intern Med 2016;55:3105-11. [Crossref] [PubMed]
- Jeyabalan A, Bhatt N, Plummeridge MJ, et al. Adequacy of endobronchial ultrasound-guided transbronchial needle aspiration samples processed as histopathological samples for genetic mutation analysis in lung adenocarcinoma. Mol Clin Oncol 2016;4:119-25. [Crossref] [PubMed]
- Fernandez-Bussy S, Labarca G, Pires Y, et al. Molecular testing of EGFR, EGFR resistance mutation, ALK and ROS1 achieved by EBUS-TBNA in Chile. Arch Bronconeumol 2017;53:172-4. [PubMed]
- Rosso L, Ferrero S, Mendogni P, et al. Ten-year experience with endobronchial ultrasound-guided transbronchial needle aspiration: single center results in mediastinal diagnostic and staging. J Thorac Dis 2017;9:S363-9. [Crossref] [PubMed]
- Sakakibara R, Inamura K, Tambo Y, et al. EBUS-TBNA as a promising method for the evaluation of tumor PD-L1 expression in lung cancer. Clin Lung Cancer 2017;18:527-34.e1. [Crossref] [PubMed]
- Raad S, Hanna N, Jalal S, et al. Endobronchial ultrasound-guided transbronchial needle aspiration use for subclassification and genotyping of lung non-small-cell carcinoma. South Med J 2018;111:484-8. [Crossref] [PubMed]
- Bellinger CR, Sharma D, Dotson T, et al. Protocol to improve genotyping of non-small-cell lung cancer diagnosed using EBUS-TBNA. South Med J 2018;111:601-6. [Crossref] [PubMed]
- Biswas A, Leon ME, Drew P, et al. Clinical performance of endobronchial ultrasound-guided transbronchial needle aspiration for assessing programmed death ligand-1 expression in nonsmall cell lung cancer. Diagn Cytopathol 2018;46:378-83. [Crossref] [PubMed]
- Fernandez-Bussy S, Pires Y, Labarca G, et al. PD-L1 expression in a non-small cell lung cancer specimen obtained by EBUS-TBNA. Arch Bronconeumol 2018;54:290-2. [PubMed]
- Ghigna MR, Crutu A, Florea V, et al. Endobronchial ultrasound-guided fine-needle aspiration for pulmonary carcinomas genotyping: experience with 398 cases including rapid EGFR/KRAS analysis in 43 cases. J Thorac Dis 2018;10:4653-8. [Crossref] [PubMed]
- Sakata KK, Midthun DE, Mullon JJ, et al. Comparison of programmed death ligand-1 immunohistochemical staining between endobronchial ultrasound transbronchial needle aspiration and resected lung cancer specimens. Chest 2018;154:827-37. [Crossref] [PubMed]
- Cicek T, Ozturk A, Yılmaz A, et al. Adequacy of EBUS-TBNA specimen for mutation analysis of lung cancer. Clin Respir J 2019;13:92-7. [Crossref] [PubMed]
- Smith A, Wang H, Zerbo A, et al. Programmed death ligand 1 testing of endobronchial ultrasound-guided transbronchial needle aspiration samples acquired for the diagnosis and staging of non-small cell lung cancer. J Bronchology Interv Pulmonol 2020;27:50-7. [Crossref] [PubMed]
- Lee YJ, Kim JH, Kim SK, et al. Lung cancer in never smokers: change of a mindset in the molecular era. Lung Cancer 2011;72:9-15. [Crossref] [PubMed]
- Labarca G, Folch E, Jantz M, et al. Adequacy of samples obtained by endobronchial ultrasound with transbronchial needle aspiration for molecular analysis in patients with non-small cell lung cancer. systematic review and meta-analysis. Ann Am Thorac Soc 2018;15:1205-16. [Crossref] [PubMed]
- Zhang Y, Xie F, Mao X, et al. Determining factors of endobronchial ultrasound-guided transbronchial needle aspiration specimens for lung cancer subtyping and molecular testing. Endosc Ultrasound 2019;8:404-11. [Crossref] [PubMed]
- Wahidi MM, Herth F, Yasufuku K, et al. Technical aspects of endobronchial ultrasound-guided transbronchial needle aspiration: CHEST guideline and expert panel report. Chest 2016;149:816-35. [Crossref] [PubMed]
- Jeyabalan A, Shelley-Fraser G, Medford AR. Impact of needle gauge on characterization of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) histology samples. Respirology 2014;19:735-9. [Crossref] [PubMed]
- Yarmus L, Akulian J, Gilbert C, et al. Optimizing endobronchial ultrasound for molecular analysis. How many passes are needed? Ann Am Thorac Soc 2013;10:636-43. [Crossref] [PubMed]
- Trisolini R, Cancellieri A, Tinelli C, et al. Randomized trial of endobronchial ultrasound-guided transbronchial needle aspiration with and without rapid on-site evaluation for lung cancer genotyping. Chest 2015;148:1430-7. [Crossref] [PubMed]
- Leong TL, Christie M, Kranz S, et al. Evaluating the genomic yield of a single endobronchial ultrasound-guided transbronchial needle aspiration in lung cancer: meeting the challenge of doing more with less. Clin Lung Cancer 2017;18:e467-72. [Crossref] [PubMed]
- Pirker R, Herth FJ, Kerr KM, et al. Consensus for EGFR mutation testing in non-small cell lung cancer: results from a European workshop. J Thorac Oncol 2010;5:1706-13. [Crossref] [PubMed]
- Tsao MS, Kerr KM, Dacic S, et al. IASLC Atlas of PDL1 immunochemistry testing in lung cancer. International Association for the Study of Lung Cancer (IASLC), 2017:1-32.
- van der Heijden EH, Casal RF, Trisolini R, et al. Guideline for the acquisition and preparation of conventional and endobronchial ultrasound-guided transbronchial needle aspiration specimens for the diagnosis and molecular testing of patients with known or suspected lung cancer. Respiration 2014;88:500-17. [Crossref] [PubMed]
- Maniwa T, Shintani Y, Okami J, et al. Upfront surgery in patients with clinical skip N2 lung cancer based on results of modern radiological examinations. J Thorac Dis 2018;10:6828-37. [Crossref] [PubMed]
- Huber RM, De Ruysscher D, Hoffmann H, et al. Interdisciplinary multimodality management of stage III nonsmall cell lung cancer. Eur Respir Rev 2019;28:190024 [Crossref] [PubMed]
- Castello A, Rossi S, Lopci E. 18F-FDG PET/CT in restaging and evaluation of response to therapy in lung cancer: state of art. Curr Radiopharm 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Cetinkaya E, Usluer O, Yılmaz A, et al. Is endobronchial ultrasound-guided transbronchial needle aspiration an effective diagnostic procedure in restaging of non-small cell lung cancer patients?. Endosc Ultrasound 2017;6:162-7. [Crossref] [PubMed]
- Genestreti G, Burgio MA, Matteucci F, et al. Endobronchial/endoesophageal ultrasound (EBUS/EUS) guided fine needle aspiration (FNA) and 18F-FDG PET/CT scanning in restaging of locally advanced non-small cell lung cancer (NSCLC) treated with chemo-radiotherapy: a mono-institutional pilot experience. Technol Cancer Res Treat 2015;14:721-7. [Crossref] [PubMed]
- Herth FJ, Annema JT, Eberhardt R, et al. Endobronchial ultrasound with transbronchial needle aspiration for restaging the mediastinum in lung cancer. J Clin Oncol 2008;26:3346-50. [Crossref] [PubMed]
- Szlubowski A, Herth FJ, Soja J, et al. Endobronchial ultrasound-guided needle aspiration in non-small-cell lung cancer restaging verified by the transcervical bilateral extended mediastinal lymphadenectomy - a prospective study. Eur J Cardiothorac Surg 2010;37:1180-4. [Crossref] [PubMed]
- Zielinski M, Szlubowski A, Kołodziej M, et al. Comparison of endobronchial ultrasound and/or endoesophageal ultrasound with transcervical extended mediastinal lymphadenectomy for staging and restaging of non-small-cell lung cancer. J Thorac Oncol 2013;8:630-6. [Crossref] [PubMed]
- Szlubowski A, Zieliński M, Soja J, et al. Accurate and safe mediastinal restaging by combined endobronchial and endoscopic ultrasound-guided needle aspiration performed by single ultrasound bronchoscope. Eur J Cardiothorac Surg 2014;46:262-6. [Crossref] [PubMed]
- Nasir BS, Bryant AS, Minnich DJ, et al. The efficacy of restaging endobronchial ultrasound in patients with non-small cell lung cancer after preoperative therapy. Ann Thorac Surg 2014;98:1008-12. [Crossref] [PubMed]
- Muthu V, Sehgal IS, Dhooria S, et al. Efficacy of endosonographic procedures in mediastinal restaging of lung cancer after neoadjuvant therapy: a systematic review and diagnostic accuracy meta-analysis. Chest 2018;154:99-109. [Crossref] [PubMed]
- Jiang L, Huang W, Liu J, et al. Endosonography with lymph node sampling for restaging the mediastinum in lung cancer: a systematic review and pooled data analysis. J Thorac Cardiovasc Surg 2020;159:1099-108.e5. [Crossref] [PubMed]
- Shingyoji M, Nakajima T, Nishimura H, et al. Restaging by endobronchial ultrasound-guided transbronchial needle aspiration in patients with inoperable advanced lung cancer. Intern Med 2010;49:787-90. [Crossref] [PubMed]
- Chiang AC, Fernandes AW, Pavilack M, et al. EGFR mutation testing and treatment decisions in patients progressing on first- or second-generation epidermal growth factor receptor tyrosine kinase inhibitors. BMC Cancer 2020;20:356. [Crossref] [PubMed]
- Chocarro de Erauso L, Zuazo M, Arasanz H, et al. Resistance to PD-L1/PD-1 blockade immunotherapy. A tumor-intrinsic or tumor-extrinsic phenomenon? Front Pharmacol 2020;11:441. [Crossref] [PubMed]
- Hsieh MS, Lin MW, Lee YH. Lung adenocarcinoma with sarcomatoid transformation after tyrosine kinase inhibitor treatment and chemotherapy. Lung Cancer 2019;137:76-84. [Crossref] [PubMed]
- Kirita K, Izumo T, Matsumoto Y, et al. Bronchoscopic re-biopsy for mutational analysis of non-small cell lung cancer. Lung 2016;194:371-8. [Crossref] [PubMed]
- Izumo T, Matsumoto Y, Chavez C, et al. Re-biopsy by endobronchial ultrasound procedures for mutation analysis of non-small cell lung cancer after EGFR tyrosine kinase inhibitor treatment. BMC Pulm Med 2016;16:106. [Crossref] [PubMed]
- Chaddha U, Hogarth DK, Murgu S. The role of endobronchial ultrasound transbronchial needle aspiration for programmed death ligand 1 testing and next generation sequencing in advanced non-small cell lung cancer. Ann Transl Med 2019;7:351. [Crossref] [PubMed]
- Kage H, Kohsaka S, Shinozaki-Ushiku A, et al. Small lung tumor biopsy samples are feasible for high quality targeted next generation sequencing. Cancer Sci 2019;110:2652-7. [PubMed]
- Xie F, Zheng X, Mao X, et al. Next-Generation sequencing for genotyping of endobronchial ultrasound-guided transbronchial needle aspiration samples in lung cancer. Ann Thorac Surg 2019;108:219-26. [Crossref] [PubMed]
- Stoy SP, Segal JP, Mueller J, et al. Feasibility of endobronchial ultrasound-guided transbronchial needle aspiration cytology specimens for next generation sequencing in non-small-cell lung cancer. Clin Lung Cancer 2018;19:230-8.e2. [Crossref] [PubMed]
- Turner SR, Buonocore D, Desmeules P, et al. Feasibility of endobronchial ultrasound transbronchial needle aspiration for massively parallel next-generation sequencing in thoracic cancer patients. Lung Cancer 2018;119:85-90. [Crossref] [PubMed]
- Fielding D, Dalley AJ, Bashirzadeh F, et al. Diff-quik cytology smears from endobronchial ultrasound transbronchial needle aspiration lymph node specimens as a source of DNA for next-generation sequencing instead of cell blocks. Respiration 2019;97:525-39. [Crossref] [PubMed]
- Inage T, Nakajima T, Itoga S, et al. Molecular nodal staging using miRNA expression in lung cancer patients by endobronchial ultrasound-guided transbronchial needle aspiration. Respiration 2018;96:267-74. [Crossref] [PubMed]
Cite this article as: Muriana P, Rossetti F. The role of EBUS-TBNA in lung cancer restaging and mutation analysis. Mediastinum 2020;4:23.


