
Growing thymic granuloma adjacent to a thymic cyst mimicking malignancy: a case report
Introduction
An association between the thymic cyst and thymic epithelial tumors has been reported (1). 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) has been widely used to differentiate benign from malignant lesions in thymic pathology (2). However, several recent case studies have reported FDG-avid granulomas in the thymus mimicking thymic malignancy (3-5). Thymic cyst rupture has been suggested as a pathogenic mechanism for inflammatory granuloma (6). However, the natural growth history of thymic granuloma remains unclear. We herein report a case of a patient who was found to have a growing nodular lesion with high FDG avidity adjacent to a long-term followed-up thymic cyst and underwent total thymectomy which revealed a granuloma in the thymus. To the best of our knowledge, this is the first reported case demonstrating the natural growth history of a thymic granuloma adjacent to a thymic cyst. We present the following case in accordance with CARE reporting checklist available at http://dx.doi.org/10.21037/med-20-46.
Case presentation
A 65-year-old man with no significant past medical history was found to have a thymic cystic nodule in a chest computed tomography (CT) scan (Figure 1A). Further workups including magnetic resonance imaging (MRI) with contrast revealed a nonenhanced thymic cystic lesion, which was suspected to be a thymic cyst. The patient was followed-up with annual CT scans, which showed a growing solid nodule adjacent to the known thymic cyst (Figure 1B). FDG-PET showed high metabolic activity with the maximum standardized uptake value (SUVmax) of 12.1 in a 2.5 cm solid mass adjacent to the cyst (Figure 1C). The timeline of follow-up evaluations and treatment for the thymic cyst and adjacent nodule is presented in Figure 1D. Chest CT scan and MRI with contrast demonstrated the innominate vein was compressed by the solid mass. We strongly suspected a thymic epithelial tumor arising on the wall of the thymic cyst with a direct invasion to the innominate vein.
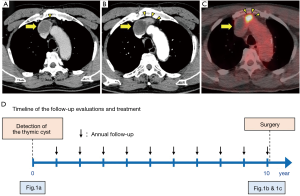
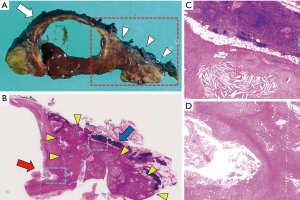
We performed a total thymectomy via median sternotomy at ten years since the first detection of the thymic cyst when the patient was 75 years old. The cyst and solid mass attached to the innominate vein but there was no direct invasion to the vascular wall. Therefore, we were able to dissect the tumor from the vein. Histopathologic examination revealed a cystic lesion with a thickened wall, as well as accumulated foam cells and cholesterol cleft granulation. A solid mass adjacent to the cyst consisted of cholesterol cleft granulation which directly connected to the thickened part of the cystic wall through a rupture of the wall (Figure 2). The postoperative course was uneventful, and the patient was followed up without evidence of recurrence for 3 months after surgery. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Discussion
In the present case, we highly suspected a malignant thymic epithelial tumor based on its growing nature (7) and high metabolic activity (2). Furthermore, we assumed that the growing nodule could be associated with the adjacent thymic cyst (1).
Previous studies have demonstrated that the growth speed of thymic epithelial tumors was associated with their histologic grade, and reported the median tumor doubling time of thymic carcinomas ranges between 146 and 205 days (7-9). In this case, the tumor doubling time was calculated to be 193 days, which suggested malignancy.
On the other hand, studies have demonstrated that there is increased FDG avidity in high-risk thymic epithelial tumors compared with low-risk tumors, thus FDG-PET is useful to differentiate anterior mediastinal lesions (2,10,11). Inoue et al. demonstrated a SUVmax of 4.5 as a useful cutoff to differentiate high-risk (WHO type B2, B3, and thymic carcinoma) from low-risk (WHO type A, AB, and B1) thymic epithelial tumors (10). In our previous study on confined early-stage (Masaoka stage I or II) thymic epithelial tumors, a SUVmax of 3.5 was an optimal cutoff value to differentiate high- and low-risk tumors (11). The present case had a SUVmax of 12.1, which also suggested malignancy.
Thymic granuloma is a rare entity and has only been presented in a few case reports (3-6), some of which demonstrated cases with high metabolic activity mimicking thymic malignancy (3-5) as in our present case. Weissferdt et al. reported four cases of cholesterol granuloma in the thymus and proposed an inflammatory response provoked by the rupture of the cyst walls leading to the formation of cholesterol cleft granuloma with foreign body-type giant cells as the pathogenesis of cholesterol granuloma in the thymus (6). Nagata et al. demonstrated granulomas in the lining of the ruptured cystic walls in the thymus (5). In the present case, the fact that a solid nodule adjacent to a thymic cyst increased in size during follow-up and the histopathologic findings as described above supported the proposed pathogenesis of thymic granuloma.
Conclusions
We report a case of growing granuloma adjacent to a thymic cyst. Although rare, a thymic granuloma should be considered as a differential diagnosis for growing, FDG-avid solid nodule in the thymus.
Acknowledgments
We would like to thank Editage (
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/med-20-46
Conflict of interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-46). The authors have no conflict of interest to be declared.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shen X, Jin Y, Shen L, et al. Thymoma and thymic carcinoma associated with multilocular thymic cyst: a clinicopathologic analysis of 18 cases. Diagn Pathol 2018;13:41. [Crossref] [PubMed]
- El-Bawab H, Al-Sugair AA, Rafay M, et al. Role of flourine-18 fluorodeoxyglucose positron emission tomography in thymic pathology. Eur J Cardiothorac Surg 2007;31:731-6. [Crossref] [PubMed]
- Zhang J, Lin X, Bai Y, et al. Thymic Granuloma Mimicking Malignancy on 18F-FDG PET/CT. Clin Nucl Med 2020;45:398-400. [Crossref] [PubMed]
- Fujimoto K, Takamori S, Yano H, et al. Focal cholesterol granuloma in the anterior mediastinum: [18F]-fluoro-2-deoxy-D-glucose-positron emission tomography and magnetic resonance imaging findings. J Thorac Oncol 2007;2:1054-6. [Crossref] [PubMed]
- Nagata S, Ishihara M, Omasa M, et al. Multifocal thymic cysts with cholesterol granuloma. Respirol Case Rep 2018;6:e00361 [Crossref] [PubMed]
- Weissferdt A, Kalhor N, Moran C. Primary thymic cholesteroloma: a clinicopathological correlation of four cases of an unusual benign lesion. Virchows Arch 2015;467:609-11. [Crossref] [PubMed]
- Jeong DY, Lee KS, Chung MJ, et al. JOURNAL CLUB: Doubling Time of Thymic Epithelial Tumors Correlates With World Health Organization Histopathologic Classification. AJR Am J Roentgenol 2017;209:W202-W210 [Crossref] [PubMed]
- Choe J, Lee SM, Lim S, et al. Doubling time of thymic epithelial tumours on CT: correlation with histological subtype. Eur Radiol 2017;27:4030-6. [Crossref] [PubMed]
- Fukumoto K, Fukui T, Kawaguchi K, et al. The tumor doubling time is a useful parameter for predicting the histological type of thymic epithelial tumors. Surg Today 2019;49:656-60. [Crossref] [PubMed]
- Inoue A, Tomiyama N, Tatsumi M, et al. (18)F-FDG PET for the evaluation of thymic epithelial tumors: Correlation with the World Health Organization classification in addition to dual-time-point imaging. Eur J Nucl Med Mol Imaging 2009;36:1219-25. [Crossref] [PubMed]
- Eguchi T, Yoshida K, Hamanaka K, et al. Utility of 18F-fluorodeoxyglucose positron emission tomography for distinguishing between the histological types of early stage thymic epithelial tumours. Eur J Cardiothorac Surg 2012;41:1059-62. [Crossref] [PubMed]
Cite this article as: Takeda T, Eguchi T, Koike S, Koyama T, Matsuoka S, Miura K, Hamanaka K, Satoh Y, Uehara T, Shimizu K. Growing thymic granuloma adjacent to a thymic cyst mimicking malignancy: a case report. Mediastinum 2020;4:28.


