
Clinical approach to childhood mediastinal tumors and management
Introduction
The mediastinum is the most common site for chest mass in childhood (1). As per etiology these cases can be divided into congenital anomalies, infectious, benign and malignant neoplasms and pseudomasses (e.g., prominent thymus) (1-3). Middle mediastinal masses, comprising 20–25% of all mediastinal masses, are further classified into vascular and non-vascular lesions. Seventy to 75 percent of mediastinal masses in childhood are malignant (4-7).
Initial presentation
The mediastinal mass might be an incidental finding on imaging when patient is being evaluated for an unrelated cause or may present with symptoms.
Mass effect
Due to compression or direct involvement of nearby structures symptoms like cough, stridor, dysphagia, hoarseness, pain, hemoptysis, shortness of breath, upper extremity and/or facial swelling (superior vena cava syndrome), hypotension (cardiac tamponade) and neurological symptoms (Horner syndrome) may occur.
Systemic symptoms
“B” symptoms such as fever, weight loss, night sweats in the case of lymphoma or symptoms due to paraneoplastic syndrome like myasthenia gravis in a patient with thymoma.
What is mediastinum and practical approach to mediastinal masses
Anatomy of mediastinum
Anatomy of the mass is useful in narrowing down the differential diagnosis and also for further planning of surgery or biopsy technique (Table 1). The mediastinum is conventionally defined as “the space between the lungs”. The borders of the mediastinum are the thoracic inlet superiorly, diaphragm inferiorly, sternum anteriorly, spine posteriorly, and pleural spaces laterally.
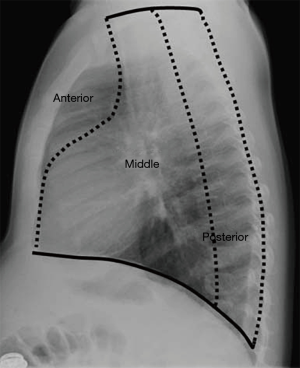
The Felson’s method divides mediastinum based on findings at lateral chest radiography into three compartments, anterior, middle and posterior (Figure 1) (8,9).
The Japanese Association for Research on the Thymus (JART) proposed the new classification of mediastinum based on transverse CT images, into four compartments: superior portion of mediastinum, anterior mediastinum (prevascular zone), middle mediastinum (peri-tracheoesophageal zone), and posterior mediastinum (paravertebral zone). Recently, International Thymic Malignancy Interest Group adopted a computed tomography (CT)-based mediastinal division into prevascular (anterior), a visceral (middle), and a paravertebral (posterior) compartment as a new standard (10,11).
Anterior mediastinal masses
The most common masses in anterior mediastinum are lymphoma, thymoma, teratoma, angiomas, lipoma and thyroid tumors (4).
Benign lesions
(I) Thymus
Primary thymus can appear prominent in infants and young children. It is difficult to distinguish it from other anterior mediastinal masses. In adolescence and adult patients normal thymus is usually not visible.
(II) Thymic cyst
It is a rare condition which presents with fluid containing mass and is remnant of the thymo-pharyngeal duct. Similarly, lymphatic malformation is a lymph containing cystic structure.
(III) Lipomas
They are encapsulated masses with content similar to subcutaneous fat. They might occur at different positions in the body including mediastinum. Patients with lipomas are largely asymptomatic. Treatment is only required only if this tumor becomes massive and shows mass effect on adjacent structures. Another commonly asymptomatic lesion is thymolipoma.
Malignant lesions
(I) Thymoma
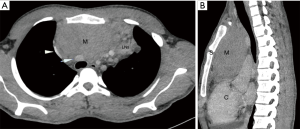
They are epithelial neoplasms containing variable number of lymphocytes. Thymomas are infrequent cause of mediastinal tumors in pediatric age group. Apart from compressive symptoms around half of patients present with paraneoplastic syndrome like hypogammaglobulinemia or myasthenia gravis (12) (Figure 2).
(II) Lymphoma
The most common cause of anterior mediastinal mass in children is lymphoma (13). They are the most common mediastinal tumor in older children. Pediatric patients present with T-lymphoblastic lymphoma (T-LBL) and classical Hodgkin’s lymphoma (CHL) have excellent prognosis with cure rates greater than 90%, in contrast to adult patients, who present with complex subtypes of T-LBL/T-cell acute lymphoblastic leukaemia (T-ALL), CHL, primary mediastinal b cell lymphoma (PMBL), diffuse large B cell lymphoma (DLBCL), and mucosa associated lymphoid tissue (MALT) lymphomas. Accurate diagnosis can be achieved with immunophenotyping and histopathologic and cytogenetic studies on acquired tissue biopsies.
(III) Germ cell tumor (GCT)
Primary mediastinal GCTs are rare and located in anterior mediastinum. They are histopathologically difficult to distinguish from testicular GCT and thus any patient who presents with mediastinal GCT needs exclusion of testicular and ovarian primary. Testicular GCTs rarely metastasize to the anterior mediastinum, so a tumor there is unlikely to be a metastasis from a testicular primary. Teratoma accounts for majority of GCT. The findings on CT or magnetic resonance imaging (MRI) in a typical teratoma is fat, fluid and calcified components. Mediastinal GCTs show a typical bimodal age distribution, with first peak in infancy and the first few years of childhood, i.e., up to 4 to 5 years of age (mainly teratoma and yolk sac), and thereafter in adolescence and young adulthood, i.e., starting at 12 to 13 years of age (seminoma) (14). Most infants have chronic cough, chest pain, wheezing, and, rarely, acute respiratory distress. In rare cases the tumor may rupture into a bronchus, resulting in expectoration of hair.
Middle mediastinal masses
As described above middle mediastinal masses for practical purpose can be divided into vascular and non-vascular lesions or as benign or malignant lesions.
Vascular lesions are double aortic arch, right aortic arch, left aortic arch with aberrant right subclavian artery, pulmonary artery sling and duplicated superior vena cava. Non-vascular lesions can be congenital foregut duplication cyst, esophageal duplication cysts and neuro-enteric cyst.
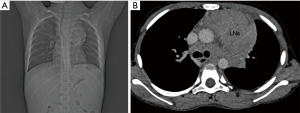
Another lesion common in middle mediastinum is lymphadenopathy which can be infectious or, more commonly of neoplastic etiology. The most common infectious cause of middle mediastinal lymphadenopathy is tuberculosis and histoplasmosis. Patients who have infectious lymphadenopathy usually have associated parenchymal abnormality aiding in the diagnosis. Neoplastic causes of middle mediastinal lymphadenopathy are lymphoma, abdominopelvic tumors like Wilms tumor, GCT and osteosarcoma. Calcification might give a clue to osteosarcoma or lymphoma (1) (Figure 3).
Posterior mediastinum masses
Sympathetic ganglion tumors
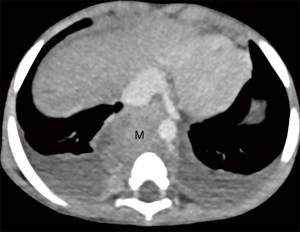
Most of the posterior mediastinal tumors are neurogenic arising from sympathetic ganglia located near thoracic vertebral bodies. Majority of tumors are neuroblastomas, which occur most often in first 3 years of life, followed by less common tumors like ganglioneuroblastomas; the rest are ganglioneuromas, occurring in older children (Figure 4).
The primary presentation of neuroblastoma within the mediastinum occurs in 11% to 26% of all neuroblastomas and present in early stage with favorable prognosis compared to those arising in abdomen (15). Thoracic neuroblastomas may not cause any symptoms and are usually diagnosed by imaging studies incidentally. Presenting symptoms may range from chronic cough to acute breathlessness. Thoracic neuroblastoma can present with Horner’s syndrome. Thoracic neuroblastoma might also cause neurologic symptoms, including weakness, limping, paralysis, and even bladder and bowel dysfunction. Some patients present with unexplained fever, weight loss, and peri-orbital ecchymosis. Similarly, presence of bone metastases can lead to bony pains and pathologic fractures (16). The presence of calcification in a posterior mediastinal tumor on chest radiography indicates a neurogenic tumor.
Initial presentation
Malignant lesions are more symptomatic in the form of malaise, unexplained fever, lymphadenopathy, neck swelling in comparison to benign lesions.
If symptoms progress very rapidly (over the course of days to weeks) then it could be lymphoblastic non-Hodgkin’s lymphoma (LB-NHL), while gradual onset might be suggestive of disease like teratoma. An intermediate progression points towards Hodgkin disease (HD) or mediastinal large cell NHL (MLC-NHL). Most thymic lesions are benign in children like thymic cyst or hyperplasia (10).
Rapidly progressive disease like LB-NHL usually present with a heterogenous mass in mediastinum and usually patients have “B” symptoms, raised lactate dehydrogenase (LDH) level and pleural effusion. Fine needle aspiration cytology (FNAC) should be done for confirmation of diagnosis. Other work up like bone marrow aspiration, biopsy, peripheral smear and pleural fluid (if present) including cytological assessment must be done. In adolescent children nonseminomatous germ cell tumor (NSGCT) is also an important differential in patients presenting with rapid onset symptoms and mediastinal mass. Tumors markers assay, as described below, must be done to rule out GCT. If symptoms occur over months to week (intermediate) HD or MLC-NHL are important differentials. Lymphoma usually presents with lymphadenopathy involving various groups of lymph nodes. HD usually presents with generalized lymphadenopathy involving cervical, abdominal and hilar regions, usually having B symptoms. MLC NHL is usually associated with mediastinal lymphadenopathy with or without pleural or/and pericardial effusion. Biopsy is required for confirmation of diagnosis. Complete staging work-up is needed after confirmation of diagnosis before starting chemotherapy. Rarely, recurrent Wilms tumor may present as a mediastinal mass or arise from a teratoma. The diagnosis requires histopathology via thoracotomy or image guided biopsy. In chronic onset symptomatic patients with anterior mediastinal mass benign teratoma is one of the differential diagnosis.
Laboratory studies
Tumor markers including beta human chorionic gonadotropin (beta-HCG), alpha-fetoprotein (AFP) and LDH should be obtained routinely. They are helpful in presumptive diagnosis in some cases of anterior mediastinal masses like thymoma or GCT. Markedly elevated AFP and HCG is pathognomic and present in more than 90% of cases of GCT. If elevated levels of beta-HCG or AFP are present along with radiological findings then immediate treatment should be started and pathological diagnosis can be skipped. AFP is elevated in non-seminomatous GCTs. Beta-HCG is associated with seminoma and non-seminomatous GCT. LDH, though non-specific, may be elevated in patients with non-seminomatous dysembryoma and lymphoma. In some patients with thymic tumors anti-cholinesterase receptor antibodies may be positive and this indicates the herald of myasthenia gravis. Urine catecholamines are elevated in neuroblastoma, however definitive diagnosis requires a biopsy along with bone marrow aspirate and biopsy. Similarly, diagnosis of osteosarcoma will require histopathological diagnosis.
Imaging
Imaging is paramount in making a differential diagnosis of mediastinal masses in pediatric population.
Contrast-enhanced CT (CECT)
It is one of the most important investigation. It shows tumor size, location, characteristics (fat/fluid/calcification) and adherences to nearby structures. This is important in deciding further planning of treatment and later in the response assessment.
MRI
It is useful in the case of large mediastinal mass when it is difficult to differentiate between compression or invasion by CT scan.
Positron emission tomography (PET)-CT
In case of lymphoma PET may be used for complete staging work-up and response assessment after treatment.
MR spine
In the case of posterior mediastinal mass MR spine can be used for evaluation of neural involvement by tumors.
Ultrasound (USG) scrotum
In case of GCT and lymphoma scrotal USG should be done to exclude a primary gonadal tumor.
Pathologic diagnostic considerations
All mediastinal masses won’t need histopathological confirmation because some show characteristics symptomatology (10). For example, benign teratoma can be diagnosed by proper history taking (chronic course) and characteristic radiological findings. In the case of thymoma if patient presents with mediastinal mass along with myasthenia gravis with other symptoms like pure red cell aplasia and hypogammaglobulinemia, and if CT scan is also suggestive of same then biopsy can be avoided for confirmation of thymic malignancy. In such a case scenario surgical intervention should be done upfront. In case of lymphoma histopathology is mandatory even if diagnosis could be made with clinical and radiological features for full characterization of tumor type and optimal treatment decisions. In lymphoma like LB-NHL FNAC is also sufficient because it shows characteristic cytological appearance. While in other types of mediastinal lymphoma biopsy is mandatory for complete characterization of tumor. Mediastinal lymphomas are usually denser and more fibrotic so biopsy is difficult and sometimes yield is not appropriate. Hence multiple samples should be taken in single attempt for histopathological evaluation and immunohistochemistry. Needle aspirate should also be taken for flow cytometry. However, the success rate of this combined core needle biopsy and aspiration approach of anterior mediastinal lymphoma is not well defined.
Various techniques are available for biopsy of mediastinal mass like image guided (CT/USG), mediastinotomy, video-assisted thoracoscopic surgery (VATS), and open surgery biopsy. Image-guided transthoracic biopsy is most commonly used. Each of the techniques has pros and cons, but decision usually depends on tumor size, location, age and performance status.
Treatment
The standard modality of treatment for thymoma is surgery. Excision of entire mass is necessary to assess extension through the capsule. Thymic carcinoma has poorer prognosis due to aggressive local growth and metastatic presentation. Surgery is the mainstay of treatment with neoadjuvant chemotherapy being utilized in advanced cases to improve the resectability of tumor followed by postoperative radiation therapy in selected cases (17).
T-LBL and B-ALL is treated with multidrug regimen protocol, intensified according to immunophenotype and risk stratification, which are divided into several phases (i.e., induction, consolidation, and maintenance) and central nervous system (CNS) directed therapy.
The current modality of treatment for lymphomas is multi-agent chemotherapy ± rituximab with or without radiation therapy. Bone marrow transplant is useful in relapsed cases in both CHL and NHL (18).
The mainstay of treatment for mature teratomas is total surgical excision. Immature teratomas are treated with chemotherapy before radical resection. After surgery, close clinical follow up along with tumor markers is important for patients with immature teratoma (14). Mediastinal seminomas are treated with cisplatin-based chemotherapy. Residual masses are treated with salvage chemotherapy or surgery depending on clinical scenario. Radiotherapy is not routinely used in pediatric population due to long term side effects and survivorship issues. Patients with mediastinal non-seminomatous GCT are treated with chemotherapy and surgery is done for residual masses (19).
For thymic cysts and lipomas surgery is often curative.
Treatment of neuroblastoma is based on tumor stage, biological properties, and is largely risk-oriented. Low-risk patients may be treated with resection alone, whereas higher-risk patients require aggressive multimodality therapy with chemotherapy, surgery, stem cell rescue, biologic/immunologic therapy and radiotherapy. The survival rate for patients with mediastinal neuroblastoma is higher than for those with abdominal tumours (15). Wilms tumor is treated with multimodal therapy (surgery, chemotherapy, and radiation). Osteosarcoma metastatic to mediastinal lymph nodes is treated with chemotherapy and surgery/radiotherapy to local site.
Conclusions
Mediastinal masses in children may be pseudomasses, benign and malignant neoplasms, congenital anomalies or infections. The diagnosis is based on symptomatology and imaging plays a paramount role along with tumor markers. A clinically sound diagnostic approach will lead to a correct diagnosis and subsequently help in optimal management.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Deepali Jain) for the series “Pediatric Mediastinal Tumors” published in Mediastinum. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-19-82). The series “Pediatric Mediastinal Tumors” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ranganath SH, Lee EY, Restrepo R, et al. Mediastinal Masses in Children. AJR Am J Roentgenol 2012;198:W197-216 [Crossref] [PubMed]
- O’Keeffe FN, Swischuk LE, Stansberry SD. Mediastinal pseudomass caused by compression of the thymus in neonates with anterior pneumothorax. AJR Am J Roentgenol 1991;156:145-8. [Crossref] [PubMed]
- Kaplan G, Sarino EF, Principato DJ. Pseudotumor of the Mediastinum. Chest 1973;63:620-1. [Crossref] [PubMed]
- Grosfeld JL, Skinner MA, Rescorla FJ, et al. Mediastinal tumors in children: Experience with 196 cases. Ann Surg Oncol 1994;1:121-7. [Crossref] [PubMed]
- Gun F, Erginel B, Ünüvar A, et al. Mediastinal Masses In Children: Experience With 120 Cases. Pediatr Hematol Oncol 2012;29:141-7. [Crossref] [PubMed]
- Simpson I, Campbell PE. Mediastinal masses in childhood: a review from a paediatric pathologist’s point of view. Prog Pediatr Surg 1991;27:92-126. [Crossref] [PubMed]
- Chen CH, Wu KH, Chao YH, et al. Clinical manifestation of pediatric mediastinal tumors, a single center experience. Medicine (Baltimore) 2019;98:e16732 [Crossref] [PubMed]
- Lee EY. Evaluation of non-vascular mediastinal masses in infants and children: an evidence-based practical approach. Pediatr Radiol 2009;39:S184-90. [Crossref] [PubMed]
- Felson B. The mediastinum. Semin Roentgenol 1969;4:41-58. [Crossref]
- Carter BW, Marom EM, Detterbeck FC. Approaching the Patient with an Anterior Mediastinal Mass: A Guide for Clinicians. J Thorac Oncol 2014;9:S102-9. [Crossref] [PubMed]
- Carter BW, Benveniste MF, Madan R, et al. ITMIG Classification of Mediastinal Compartments and Multidisciplinary Approach to Mediastinal Masses. Radiographics 2017;37:413-36. [Crossref] [PubMed]
- Spigland N, Lorenzo MD, Youssef S, et al. Malignant thymoma in children: A 20-year review. J Pediatr Surg 1990;25:1143-6. [Crossref] [PubMed]
- Glick RD, La Quaglia MP. Lymphomas of the Anterior Mediastinum. Semin Pediatr Surg 1999;8:69-77. [Crossref] [PubMed]
- Yalçın B, Demir HA, Tanyel FC, et al. Mediastinal germ cell tumors in childhood. Pediatr Hematol Oncol 2012;29:633-42. [Crossref] [PubMed]
- Suita S, Tajiri T, Sera Y, et al. The characteristics of mediastinal neuroblastoma. Eur J Pediatr Surg 2000;10:353-9. [Crossref] [PubMed]
- Malek MM, Mollen KP, Kane TD, et al. Thoracic neuroblastoma: a retrospective review of our institutional experience with comparison of the thoracoscopic and open approaches to resection. J Pediatr Surg 2010;45:1622-6. [Crossref] [PubMed]
- Stachowicz-Stencel T, Orbach D, Brecht I, et al. Thymoma and thymic carcinoma in children and adolescents: A report from the European Cooperative Study Group for Pediatric Rare Tumors (EXPeRT). Eur J Cancer 2015;51:2444-52. [Crossref] [PubMed]
- Allen CE, Kelly KM, Bollard CM. Pediatric Lymphomas and Histiocytic Disorders of Childhood. Pediatr Clin North Am 2015;62:139-65. [Crossref] [PubMed]
- Bokemeyer C, Nichols CR, Droz JP, et al. Extragonadal germ cell tumors of the mediastinum and retroperitoneum: results from an international analysis. J Clin Oncol 2002;20:1864-73. [Crossref] [PubMed]
Cite this article as: Verma S, Kalra K, Rastogi S, Sidhu HS. Clinical approach to childhood mediastinal tumors and management. Mediastinum 2020;4:21.



