
The impact of pathological analysis on endobronchial ultrasound diagnostic accuracy
Introduction
Lung cancer is the leading cause of tumor related mortality (1). Early diagnosis, assessment of nodal status and acquisition of adequate tissue for cytological and histological subtyping and genotyping are essential in lung cancer workup (2). Endobronchial ultrasound transbronchial needle aspiration (EBUS-TBNA) is a minimally invasive procedure, which has emerged as a technique for hilar and mediastinal lymph node diagnosis and staging (3,4). It provides real time access under echographic guidance to paratracheal, subcarinal, hilar, interlobar and lobar lymph nodes and allows the collection of specimens for diagnosis and ancillary testing (5,6). EBUS-TBNA is an accurate, cost-effective and safe procedure with a low morbidity rate, and therefore the American College of Chest Physicians and the European Society of Thoracic Surgeons recommend it as the procedure of choice for invasive mediastinal staging (7-11). Reported data concerning diagnostic yield show heterogeneous results, varying from 71% to 99%, influenced by several factors as lesion size, number of lesions sampled, needle size and number of needle passes (12-16). Another issue that may influence diagnostic accuracy of EBUS-TBNA is pathological analysis. There are almost three methods of specimen preparation: cytological slides, cell-blocks and core biopsies. The choice of one processing method instead of another or their combination differs according to the single Institute preference (17,18). The use of rapid on-site evaluation (ROSE) may also influence the diagnostic yield of EBUS-TBNA. ROSE is a technique for immediate evaluation of samples obtained with EBUS-TBNA. A cytopathologist performs a rapid stain of the collected samples for an immediate evaluation, with the purpose of defining adequacy and when possible a preliminary diagnosis. ROSE can consequently guide EBUS-TBNA suggesting if the collection of further material is required. The aims of ROSE are to increase sampling adequacy, improve diagnostic yield of EBUS-TBNA and ensure sampling of adequate material for ancillary studies (5).
Specimen collection and preparation
Advances in lung cancer treatment and the possibility of tailored therapies according to specific biomarkers in the tumor cells or tissue has increased the number of minimally invasive procedures, which have progressively become more challenging both concerning the sites of the biopsies and the amount of tissue to be collected to obtain primary diagnosis, immunohistochemistry and molecular studies (19).
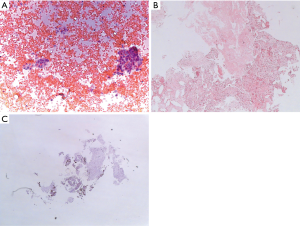
The material collected with EBUS-TBNA may be sampled and processed by several techniques: cytological smears, cell blocks or core biopsies. The EBUS-TBNA sample is immediately transferred over a glass slide and smeared with other glass slides for cytological examination. The material may be processed by air drying and wet fixation. The dried slides are stained for ROSE while wet fixed slides are stained in the cytology laboratory. The material collected and needle rinses may also be prepared as cell blocks. Tissue fragments are generally collected and fixed in 10% formalin for histological examination. As discussed by van der Heijden and colleagues, there is no consensus in the literature on the processing method associated with the best diagnostic yield (17). In fact, the studies which compared cytology smears, cell blocks and core biopsies did not find differences in terms of diagnostic accuracy (18,20,21). The specimen preparation may therefore vary between Institutions according to the expertise of the operator and the pathologist. Figure 1 depicts the cytological and histological samples of a lymph nodal localization of adenocarcinoma obtained by means of EBUS-TBNA.
Another point to be discussed concerns the role of ROSE during EBUS-TBNA. During ROSE several smears are processed with rapid staining. The type of rapid stain methods does not seem to change the results of EBUS-TBNA in terms of final diagnosis (22). After each pass a cytopathologist examines the specimen under light microscopy and evaluates its adequacy for diagnosis and ancillary studies. In case of suspicion or diagnosis of lung cancer the cytopathologist may guide the procedure requiring other passes for further sampling.
ROSE requires the availability of a cytopathologist in the endoscopic suite or in the operating room during EBUS-TBNA, which is not always possible in all Institutions because of costs, time associated to the procedure and workload for the pathology laboratories. In order to overcome these limitations in several institutions ROSE may be performed by other trained professionals like biomedical scientists or through a telecytology service in which the cytopathologist is not on site and evaluates from a remote location slide images transmitted through a digital camera connected to the microscope (23,24). However, at present these options are not commonly used since they require the training of a specific staff and the use of expensive technologies.
Type of needles and number of passes
EBUS-TBNA allows sampling of cytological and histological specimens by the use of either 22-gauge or 21-gauge needles (25). Only few studies investigated the impact of needle size on diagnostic yield of EBUS-TBNA (Table 1) (26-30). These studies did not demonstrate a clear superiority of one needle in comparison with the other. In fact, no differences in diagnostic yield were observed, even if one of the studies demonstrated that fewer needle passes were required when the 21-gauge needle was associated with ROSE (29). Jeyabalan et al. also observed a better characterization of non-small cell lung cancer with the use of a 21-gauge rather when the specimens were collected with 22-gauge needles (30). Conversely, a larger size needle may be associated with haemorrhagic contamination (26). The literature does not recommend therefore one needle instead of another and the operator may choose needle size according to site, size and vascularization of the target. Larger needles may be useful in collecting tissue fragments for histological evaluation but smaller needles may be more flexible and consequently more maneuverable in case of sampling of difficult targets (5).

Full table
A single insertion of the needle in the target lymph-node is defined as a pass, and each pass includes 5 to 15 needle excursions within the target lymph node (5). In lung cancer diagnosis and staging, the recommended number of passes at each target is a minimum of three (11,17,25,31). A study by Yarmus et al. indicates that a median of four passes is required to sample sufficient material for molecular analysis (32).
In a recent meta-analysis Sehgal et al. showed that the use of ROSE did not improve the diagnostic yield of EBUS-TBNA, and it did not reduce the procedure length (33). On the other hand, ROSE was associated with fewer number of passes and a lower number of additional bronchoscopic procedures to obtain a final diagnosis. These results are similar to those reported in the review by van der Heijden et al. in which ROSE was not associated with a better diagnostic yield of EBUS-TBNA and did not influence the duration of the procedure (17).
However, other studies showed that ROSE, when used in cases of suspected lung cancer, may reduce the number of repeated diagnostic invasive procedures, especially those aimed at molecular profiling, increasing by 10% the success rate of the procedure for genotyping (34,35).
The impact of ROSE in diagnosis, staging and molecular profiling of lung cancer
The impact of ROSE during EBUS-TBNA for diagnosis and staging of lung cancer is still a matter for debate. The main advantages of ROSE consist in increasing the diagnostic yield of the collected samples, potentially reducing the number of needle passes required to ensure adequate material for molecular profiling (36). Some authors showed an increase in diagnostic yield when ROSE is associated to CT guided fine needle aspiration, or during esophageal endoscopic ultrasound (37,38). These results have not been definitively established when ROSE is used in association with EBUS-TBNA for lung cancer diagnosis and staging (39). In fact, several studies investigated the contribution of ROSE to the adequacy of sampling for lung cancer diagnosis during EBUS-TBNA, with contradictory results (2,6,15,35,40). Some of the trials reported a significant contribution of ROSE to the diagnosis and staging of lung cancer and a high concordance between ROSE and the final diagnosis (6,41).On the contrary, randomized trials failed in finding differences in diagnostic yield when EBUS-TBNA is performed with or without the use of ROSE (33,34,42).
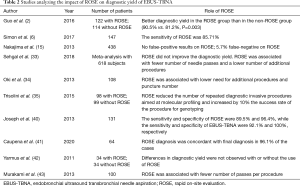
In a study by Joseph et al. conducted on a series of 131 patients, ROSE was inadequate in 30 cases, but in 22 out these 30 cases a diagnosis was obtained after the final histological analysis (40). The sensitivity and specificity of ROSE were 89.5% and 96.4%, while the sensitivity and specificity of EBUS-TBNA were 92.1% and 100%, respectively. Analyzing these data the authors concluded that ROSE did not have an impact on clinical decision making after a complete mediastinal staging with EBUS-TBNA. On the other hand, several studies reported that ROSE reduces the number of passes per procedure (33,34,42,43). Oki et al. did not find differences in EBUS-TBNA duration between ROSE and non-ROSE procedures; procedure time was 22.3 minutes when ROSE was used and 22.1 without the support of ROSE (34). As discussed by Caupena et al., the absence of differences in the duration of the procedures may be due to a reduction of the number of sampled nodes and passes per target associated with the use of ROSE. In fact, when following ROSE a diagnosis of positive N3 node is reached, no further sampling on N2/N1 sampling has necessarily to be performed (41). Moreover, without ROSE a minimum of three passes per target is advised (44), while the use of ROSE may allow to reduce the number of biopsies required to obtain diagnosis (35). In Table 2 studies concerning the use of ROSE are reported.
Full table
Other studies showed that ROSE reduces the number of additional diagnostic invasive procedures (34,35). Avoiding repeated procedures is very attractive, and Diette et al. showed that the diagnostic yield of sampling could be increased when ROSE is used to guide the number of biopsies (45). Nevertheless, the results of a study by Joseph et al. did not show a role of ROSE in avoiding unnecessary procedures. These authors therefore concluded that ROSE is not of help in decision making when complete mediastinal staging is performed with EBUS-TBNA (40).
Another point of discussion is the role of ROSE in ancillary studies and molecular profiling of lung cancer (5). This issue is particularly relevant in a clinical scenario where most patients affected by lung cancer get a diagnosis in an advanced stage (46,47). The yield of EBUS-TBNA for molecular analyses varies between 72% and 98% (21,48-59). Molecular testing accuracy may be influenced by several factors, like cellularity and tumor burden in the sample, method of fixation and the molecular platform used for the evaluation (5). ROSE may ensure the collection of adequate material for molecular profiling, including EGFR and KRAS mutations and ALK and ROS1 rearrangement (21,32,35,52). In a randomized controlled trial by Trisolini et al., molecular profiling was completed with EBUS-TBNA in more than 80% of cases (35). In this study the use of ROSE increased the success rate up to 90%. These data showed only a statistical significance trend, but the clinical impact of avoiding further invasive procedures may be considered relevant in a context where patients are often old, frail and affected by other comorbidities. In this series the use of ROSE also reduced the number of samples with a minimal tumor burden, adequate only for diagnosis and not for genotyping. If ROSE is available sampling strategy may be modified by proceeding with further sample collection when minimal tumor cells burden, necrosis or contamination with blood are observed.
Available data are inadequate to establish the precise number of passes for complete genotyping, but ROSE may be useful to direct the recruitment of additional material. Yarmus et al. in a study evaluating the number of needle passes for analyzing EGFR, KRAS and ALK showed a 95% rate of success with a median of four passes using EBUS in association with ROSE (32). The randomized controlled trial by Trisolini et al. supported the observation that four passes may be sufficient for subtyping and genotyping lung cancer (35).
The minimal impact that ROSE has on diagnostic yield in EBUS-TBNA is probably related to the high rate of success of EBUS-TBNA. This statement is supported by a meta-analysis on influence of ROSE on the adequacy rate of fine needle cytology; the impact of ROSE was minimal in the procedures characterized by a high diagnostic yield (36).
Conclusions
Several factors may influence EBUS-TBNA accuracy in lung cancer diagnosis and staging. Among these factors, pathological aspects may play a relevant role. Thanks to the improvement of lung cancer knowledge a higher number of minimally invasive diagnostic procedures has progressively become essential in diagnostic work-up with an increasing request of cytological and histological material for precise subtyping and genotyping. Considering that most patients have an advanced disease a fast, accurate and precise diagnosis is mandatory.
Concerning the pathological impact on EBUS-TBNA diagnostic accuracy, data in the literature are still controversial. Among the different techniques for processing the collected material, the superiority of one method rather than another has not been demonstrated. The guidelines for the acquisition and preparation of EBUS-TBNA specimens reflect the variability of the results and conclude that the specimen processing techniques may vary between Institutions according to the ability and expertise of the pathological staff (17).
No consensus has also been reached concerning the optimal size of the biopsy needles. The use of larger needles has been associated with a better characterization of cancer subtypes but also with a higher risk of blood contamination of the specimen (26-30). Thus, the type of needle should be chosen by the operator considering the site and the characteristics of the target.
ROSE associated with EBUS-TBNA is a valid diagnostic tool which may improve the diagnostic yield of the procedure. ROSE is effective in reducing the number of redo-procedures while does not significantly influence diagnostic yield, length of the procedure and morbidity (17).
Several advantages have been described with the use of ROSE. It warrants the adequacy of the sample and can improve the diagnostic yield, by reducing the rate of inadequate samples (60). Moreover, it can reduce the need of additional samples for molecular testing allowing an earlier conclusion of the procedure once adequate material for genotyping has been observed. Another advantage of ROSE is represented by the reduction of the total workload of the pathologic laboratory due to an overall reduction of slides to be examined.
Some authors do not consider ROSE a necessary tool during EBUS-TBNA, since during the procedure it is possible to perform a real-time check of the insertion of the needle in the target lesion, and since ROSE is a time consuming procedure. Moreover, ROSE requires a dedicated cytopathologist on site during the procedure (5). Moreover, several studies failed to demonstrate a significant advantage with the use of ROSE in case of a high EBUS-TBNA diagnostic yield (33,34,42).
EBUS-TBNA is a procedure with a high accuracy rate. The choice of specimen processing techniques and of the type of needle and the availability of ROSE may contribute to a further improvement of its results. The possibility to avoid additional invasive procedure is an important advantage, reflecting an overall improvement of patients’ care. Further studies are needed to investigate and strengthen the role of these issues and their impact in lung cancer work-up.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Angelo Carretta) for the series “Minimally Invasive (Endoscopic) Mediastinal Staging of Lung Cancer” published in Mediastinum. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-28). The series “Minimally Invasive (Endoscopic) Mediastinal Staging of Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v1-v27. [Crossref] [PubMed]
- Guo H, Liu S, Guo J, et al. Rapid on-site evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of hilar and mediastinal lymphadenopathy in patients with lung cancer. Cancer Lett 2016;371:182-6. [Crossref] [PubMed]
- Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e142S-e165S.
- Kinsey CM, Arenberg DA. Endobronchial ultrasound-guided transbronchial needle aspiration for non-small cell lung cancer staging. Am J Respir Crit Care Med 2014;189:640-9. [Crossref] [PubMed]
- Jain D, Allen TC, Aisner DL, et al. Rapid On-Site Evaluation of Endobronchial Ultrasound-Guided Transbronchial Needle Aspirations for the Diagnosis of Lung Cancer: A Perspective From Members of the Pulmonary Pathology Society. Arch Pathol Lab Med 2018;142:253-62. [Crossref] [PubMed]
- Şimon M, Pop B, Toma IL, et al. The use of EBUS-TBNA and ROSE in the diagnosis of lung cancer. Rom J Morphol Embryol 2017;58:79-87. [PubMed]
- Eapen GA, Shah AM, Lei X, et al. Complications, consequences, and practice patterns of endobronchial ultrasound-guided transbronchial needle aspiration: Results of the AQuIRE registry. Chest 2013;143:1044-53. [Crossref] [PubMed]
- Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer 2009;45:1389-96. [Crossref] [PubMed]
- Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J 2009;33:1156-64. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-e250S.
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Lee BE, Kletsman E, Rutledge JR, et al. Utility of endobronchial ultrasound-guided mediastinal lymph node biopsy in patients with non-small cell lung cancer. J Thorac Cardiovasc Surg 2012;143:585-90. [Crossref] [PubMed]
- Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 2011;142:1393-400.e1. [Crossref] [PubMed]
- Tian Q, Chen LA, Wang RT, et al. The reasons of false negative results of endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of intrapulmonary and mediastinal malignancy. Thorac Cancer 2013;4:186-90. [Crossref] [PubMed]
- Nakajima T, Yasufuku K, Saegusa F, et al. Rapid on-site cytologic evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for nodal staging in patients with lung cancer. Ann Thorac Surg 2013;95:1695-99. [Crossref] [PubMed]
- Figueiredo VR, Cardoso PF, Jacomelli M, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for lung cancer staging: early experience in Brazil. J Bras Pneumol 2015;41:23-30. [Crossref] [PubMed]
- van der Heijden EH, Casal RF, Trisolini R, et al. Guideline for the acquisition and preparation of conventional and endobronchial ultrasound-guided transbronchial needle aspiration specimens for the diagnosis and molecular testing of patients with known or suspected lung cancer. Respiration 2014;88:500-17. [Crossref] [PubMed]
- Nardecchia E, Cattoni M, Dominioni L. Endobronchial ultrasound-transbronchial needle aspiration for mediastinal staging of non-small cell lung cancer: variability of results and perspectives. J Thorac Dis 2017;9:S418-24. [Crossref] [PubMed]
- Coley SM, Crapanzano JP, Saqi A. FNA, core biopsy, or both for the diagnosis of lung carcinoma: Obtaining sufficient tissue for a specific diagnosis and molecular testing. Cancer Cytopathol 2015;123:318-26. [Crossref] [PubMed]
- Rotolo N, Cattoni M, Crosta G, et al. Comparison of multiple techniques for endobronchial ultrasound-transbronchial needle aspiration specimen preparation in a single institution experience. J Thorac Dis 2017;9:S381-5. [Crossref] [PubMed]
- Navani N, Brown JM, Nankivell M, et al. Suitability of endobronchial ultrasound-guided transbronchial needle aspiration specimens for subtyping and genotyping of non-small cell lung cancer: a multicenter study of 774 patients. Am J Respir Crit Care Med 2012;185:1316-22. [Crossref] [PubMed]
- Louw M, Brundyn K, Schubert PT, et al. Comparison of the quality of smears in transbronchial fine-needle aspirates using two staining methods for rapid on-site evaluation. Diagn Cytopathol 2012;40:777-81. [Crossref] [PubMed]
- Schacht MJ, Toustrup CB, Madsen LB, et al. Endobronchial ultrasound-guided transbronchial needle aspiration: performance of biomedical scientists on rapid on-site evaluation and preliminary diagnosis. Cytopathology 2016;27:344-50. [Crossref] [PubMed]
- Bott MJ, James B, Collins BT, et al. A Prospective Clinical Trial of Telecytopathology for Rapid Interpretation of Specimens Obtained During Endobronchial Ultrasound-Fine Needle Aspiration. Ann Thorac Surg 2015;100:201-5; discussion 205-6. [Crossref] [PubMed]
- Wahidi MM, Herth F, Yasufuku K, et al. Technical Aspects of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: CHEST Guideline and Expert Panel Report. Chest 2016;149:816-35. [Crossref] [PubMed]
- Nakajima T, Yasufuku K, Takahashi R, et al. Comparison of 21-gauge and 22-gauge aspiration needle during endobronchial ultrasound-guided transbronchial needle aspiration. Respirology 2011;16:90-4. [Crossref] [PubMed]
- Oki M, Saka H, Kitagawa C, et al. Randomized Study of 21-gauge Versus 22-gauge Endobronchial Ultrasound-guided Transbronchial Needle Aspiration Needles for Sampling Histology Specimens. J Bronchology Interv Pulmonol 2011;18:306-10. [Crossref] [PubMed]
- Saji J, Kurimoto N, Morita K, et al. Comparison of 21-gauge and 22-gauge Needles for Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration of Mediastinal and Hilar Lymph Nodes. J Bronchology Interv Pulmonol 2011;18:239-46. [Crossref] [PubMed]
- Yarmus LB, Akulian J, Lechtzin N, et al. Comparison of 21-gauge and 22-gauge aspiration needle in endobronchial ultrasound-guided transbronchial needle aspiration: results of the American College of Chest Physicians Quality Improvement Registry, Education, and Evaluation Registry. Chest 2013;143:1036-43. [Crossref] [PubMed]
- Jeyabalan A, Shelley-Fraser G, Medford AR. Impact of needle gauge on characterization of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) histology samples. Respirology 2014;19:735-9. [Crossref] [PubMed]
- Lee HS, Lee GK, Lee HS, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: how many aspirations per target lymph node station? Chest 2008;134:368-74. [Crossref] [PubMed]
- Yarmus L, Akulian J, Gilbert C, et al. Optimizing endobronchial ultrasound for molecular analysis. How many passes are needed?. Ann Am Thorac Soc 2013;10:636-43. [Crossref] [PubMed]
- Sehgal IS, Dhooria S, Aggarwal AN, et al. Impact of Rapid On-Site Cytological Evaluation (ROSE) on the Diagnostic Yield of Transbronchial Needle Aspiration During Mediastinal Lymph Node Sampling: Systematic Review and Meta-Analysis. Chest 2018;153:929-38. [Crossref] [PubMed]
- Oki M, Saka H, Kitagawa C, et al. Rapid on-site cytologic evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for diagnosing lung cancer: a randomized study. Respiration 2013;85:486-92. [Crossref] [PubMed]
- Trisolini R, Cancellieri A, Tinelli C, et al. Randomized Trial of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration With and Without Rapid On-site Evaluation for Lung Cancer Genotyping. Chest 2015;148:1430-7. [Crossref] [PubMed]
- Schmidt RL, Witt BL, Lopez-Calderon LE, et al. The influence of rapid onsite evaluation on the adequacy rate of fine-needle aspiration cytology: a systematic review and meta-analysis. Am J Clin Pathol 2013;139:300-8. [Crossref] [PubMed]
- Fassina A, Corradin M, Zardo D, et al. Role and accuracy of rapid on-site evaluation of CT-guided fine needle aspiration cytology of lung nodules. Cytopathology 2011;22:306-12. [Crossref] [PubMed]
- Tournoy KG, Praet MM, Van Maele G, et al. Esophageal endoscopic ultrasound with fine-needle aspiration with an on-site cytopathologist: high accuracy for the diagnosis of mediastinal lymphadenopathy. Chest 2005;128:3004-9. [Crossref] [PubMed]
- Griffin AC, Schwartz LE, Baloch ZW. Utility of on-site evaluation of endobronchial ultrasound-guided transbronchial needle aspiration specimens. Cytojournal 2011;8:20. [Crossref] [PubMed]
- Joseph M, Jones T, Lutterbie Y, et al. Rapid on-site pathologic evaluation does not increase the efficacy of endobronchial ultrasonographic biopsy for mediastinal staging. Ann Thorac Surg 2013;96:403-10. [Crossref] [PubMed]
- Caupena C, Esteban L, Jaen A, et al. Concordance Between Rapid On-Site Evaluation and Final Cytologic Diagnosis in Patients Undergoing Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for Non-Small Cell Lung Cancer Staging. Am J Clin Pathol 2020;153:190-7. [PubMed]
- Yarmus L, Van der Kloot T, Lechtzin N, et al. A randomized prospective trial of the utility of rapid on-site evaluation of transbronchial needle aspirate specimens. J Bronchology Interv Pulmonol 2011;18:121-7. [Crossref] [PubMed]
- Murakami Y, Oki M, Saka H, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of small cell lung cancer. Respir Investig 2014;52:173-8. [Crossref] [PubMed]
- Diacon AH, Schuurmans MM, Theron J, et al. Transbronchial needle aspirates: how many passes per target site? Eur Respir J 2007;29:112-6. [Crossref] [PubMed]
- Diette GB, White P Jr, Terry P, et al. Utility of on-site cytopathology assessment for bronchoscopic evaluation of lung masses and adenopathy. Chest 2000;117:1186-90. [Crossref] [PubMed]
- Askoxylakis V, Thieke C, Pleger ST, et al. Long-term survival of cancer patients compared to heart failure and stroke: a systematic review. BMC Cancer 2010;10:105. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Nakajima T, Yasufuku K, Suzuki M, et al. Assessment of epidermal growth factor receptor mutation by endobronchial ultrasound-guided transbronchial needle aspiration. Chest 2007;132:597-602. [Crossref] [PubMed]
- Garcia-Olivé I, Monsó E, Andreo F, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for identifying EGFR mutations. Eur Respir J 2010;35:391-5. [Crossref] [PubMed]
- Schuurbiers OC, Looijen-Salamon MG, Ligtenberg MJ, et al. A brief retrospective report on the feasibility of epidermal growth factor receptor and KRAS mutation analysis in transesophageal ultrasound- and endobronchial ultrasound-guided fine needle cytological aspirates. J Thorac Oncol 2010;5:1664-7. [Crossref] [PubMed]
- Sakairi Y, Nakajima T, Yasufuku K, et al. EML4-ALK fusion gene assessment using metastatic lymph node samples obtained by endobronchial ultrasound-guided transbronchial needle aspiration. Clin Cancer Res 2010;16:4938-45. [Crossref] [PubMed]
- Jurado J, Saqi A, Maxfield R, et al. The efficacy of EBUS-guided transbronchial needle aspiration for molecular testing in lung adenocarcinoma. Ann Thorac Surg 2013;96:1196-202. [Crossref] [PubMed]
- Folch E, Yamaguchi N, VanderLaan PA, et al. Adequacy of lymph node transbronchial needle aspirates using convex probe endobronchial ultrasound for multiple tumor genotyping techniques in non-small-cell lung cancer. J Thorac Oncol 2013;8:1438-44. [Crossref] [PubMed]
- Neat MJ, Foot NJ, Hicks A, et al. ALK rearrangements in EBUS-derived transbronchial needle aspiration cytology in lung cancer. Cytopathology 2013;24:356-64. [Crossref] [PubMed]
- Kanaji N, Bandoh S, Ishii T, et al. Detection of EML4-ALK fusion genes in a few cancer cells from transbronchial cytological specimens utilizing immediate cytology during bronchoscopy. Lung Cancer 2012;77:293-8. [Crossref] [PubMed]
- Esterbrook G, Anathhanam S, Plant PK. Adequacy of endobronchial ultrasound transbronchial needle aspiration samples in the subtyping of non-small cell lung cancer. Lung Cancer 2013;80:30-4. [Crossref] [PubMed]
- Reynolds JP, Tubbs RR, Minca EC, et al. EGFR mutational genotyping of liquid based cytology samples obtained via fine needle aspiration (FNA) at endobronchial ultrasound of non-small cell lung cancer (NSCLC). Lung Cancer 2014;86:158-63. [Crossref] [PubMed]
- Cai G, Wong R, Chhieng D, et al. Identification of EGFR mutation, KRAS mutation, and ALK gene rearrangement in cytological specimens of primary and metastatic lung adenocarcinoma. Cancer Cytopathol 2013;121:500-7. [Crossref] [PubMed]
- Nakajima T, Yasufuku K, Nakagawara A, et al. Multigene mutation analysis of metastatic lymph nodes in non-small cell lung cancer diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration. Chest 2011;140:1319-24. [Crossref] [PubMed]
- Collins BT, Chen AC, Wang JF, et al. Improved laboratory resource utilization and patient care with the use of rapid on-site evaluation for endobronchial ultrasound fine-needle aspiration biopsy. Cancer Cytopathol 2013;121:544-51. [Crossref] [PubMed]
Cite this article as: Bandiera A, Arrigoni G. The impact of pathological analysis on endobronchial ultrasound diagnostic accuracy. Mediastinum 2020;4:19.


