
Case presentations and recommendations from the 2018 ITMIG Annual Meeting
Introduction
The International Thymic Malignancy Interest Group (ITMIG) is an academic society that holds periodic tumor boards that consist of an international panel of experts with significant interest and experience in thymic malignancies, with at least one representative from the following specialties; thoracic surgeon, medical oncologist, radiation oncologist, diagnostic radiologist, and thoracic pathologist. Any clinician anywhere in the world who seeks assistance from the panel is encouraged to participant by submitting a case (including history, clinical questions, and radiographic and histologic images). Following the case discussion, the tumor board summarizes its conclusions in writing for the treating clinician to help guide them in their treatment plan. We present three cases discussed at our 9th annual meeting in Seoul, South Korea.
Case presentation 1
A previously healthy 46-year-old man experienced mild trauma to the sternum resulting in chest pain that did not resolve. He had a 9-pack year history of smoking but quit 15 years previously.
Imaging
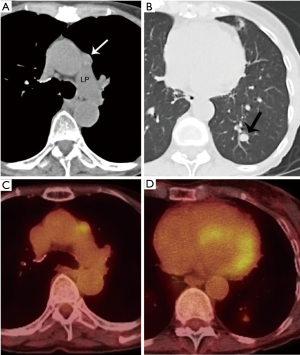
He obtained a chest radiograph (not shown). This led to a chest computed tomography scan (CT) which demonstrated a lobulated prevascular mass that abutted the pericardium (Figure 1A), with no pleural metastatic deposits and no lymphadenopathy. However, there was a region of cortical destruction of the sternum (Figure 1B) which was concerning for a bone metastasis. Due to the aggressive features of what looked like a primary mediastinal mass a whole body 18-fluorodeoxyglucose positron emission tomography (FDG PET)-CT scan was performed to assess for any other metastatic foci.
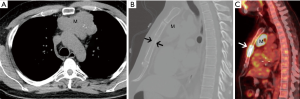
This demonstrated marked FDG uptake in the primary mediastinal mass with an SUV max of 19.5, as well as marked FDG uptake in the sternal metastasis with no other FDG avid foci of metastatic disease (Figure 1C). The differential diagnoses for a primary malignancy in the prevascular mediastinum in a 46-year-old man with marked FDG avidity include: thymic epithelial malignancy, lymphoma, or a germ cell tumor. Untreated lymphoma does not present with calcifications in the mass. It would be unusual for a germ cell tumor to present with a solitary bone metastasis as they usually metastasize to the lungs. Although most thymomas do not present with such high FDG avidity, thymic carcinoma does. In addition, most thymic carcinomas present at a later stage and the bone is one of the possible metastatic sites.
Pathology
A pre-treatment core biopsy was required as upfront surgical resection was not deemed achievable (1). Microscopy revealed fragments of fibrosis with nests of a malignant neoplasm composed of epithelioid and spindled cells. On immunostaining the tumor cells were strongly positive for CK5/6, p40, and MNF116; weakly positive for KIT; and negative for CD5 and TTF1. The associated T lymphocytes were positive for CD3 and CD5, and negative for TdT and CD1a.
Diagnosis
The morphological features and immunostaining results supported the diagnosis of metastatic non-keratinizing squamous cell carcinoma (SCC). Despite the absence of CD5 expression by the tumor cells, this was thought to represent a thymic carcinoma given the history of thymic epithelial neoplasm. About 20–40% of thymic carcinomas do not express KIT (CD117) and CD5. In these cases, correlation with the clinical setting is recommended (2). The members of the tumor board discussed that it is difficult to rule out a lung primary. Clinical staging for a thymic tumor based on imaging and biopsy is Tumor-Node-Metastasis (TNM) T3 N0 M1b (Masaoka-Koga stage IVB due to the sternal metastasis) (2).
Further work up
An echocardiogram evaluated the pericardial fluid and pericardium to rule out involvement by tumor, and they were not felt to be suspicious. The tumor board radiologist noted a CT with contrast would not detect pericardial involvement, but an MRI could have been considered.
Management
The treatment strategy for thymic epithelial tumors is primarily based on whether the tumor can be resected up front as determined by the treating surgeon, possibly with discussion in a multidisciplinary tumor board setting (2). The ITMIG thoracic oncologist agreed the tumor did not appear to be amenable to an upfront R0 resection. For Masaoka-Koga stage IVB tumors induction chemotherapy is the first part of a curative-intent sequential strategy that is followed by surgery if the tumor becomes resectable, and then postoperative radiotherapy (PORT) (2). The patient was treated with 3 cycles of the preferred induction chemotherapy regimen consisting of cisplatin, doxorubicin, and cyclophosphamide (CAP) (2), followed by an R0 resection of the sternum, involved ribs, and partial chest wall. Sampling of the N1 nodal region was completed but not the deeper N2 region, and adjuvant external beam radiotherapy (EBRT) to a dose of 50 Gray (Gy) was planned.
Imaging after induction chemotherapy
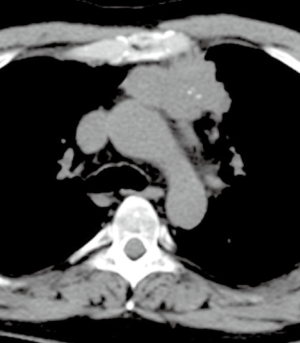
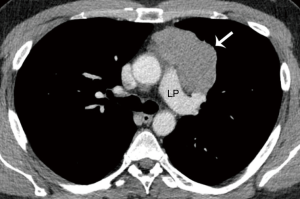
A CT scan following neoadjuvant therapy showed a partial response with a decrease of the primary mass from 9.3 cm to 6.1 cm in diameter, and the bone metastasis had become sclerotic as a response (Figure 2). No new metastatic foci were demonstrated.
Surgical pathology
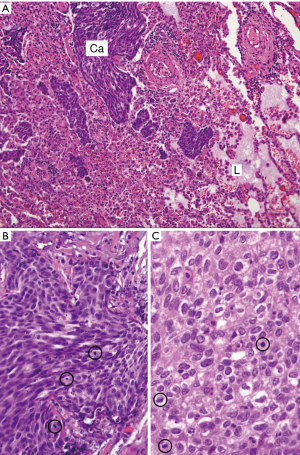
Bone of sternum and adjacent soft tissues were focally involved by carcinoma, showing prominent stromal fibrosis. The resection margins were free of tumor. The ribs were not involved, and resection margins of the ribs were free of tumor. The prevascular mass was resected with adjacent pericardium and lung wedge resection that demonstrated non-keratinizing SCC invading into the adjacent lung parenchyma (Figure 3A,B,C) and into the pericardium. The inked surgical margins of the lung and pericardium, as well as peripheral (radial) margins, were not involved. The pathology TNM staging therefore confirmed pT3 N0 M1b due to lung and chest wall involvement and the positive pulmonary intraparenchymal nodule (2).
Adjuvant radiotherapy
Adjuvant EBRT was planned to 50 Gy targeted to the tumor bed with the clinical target volume including the whole thymic space to improve local control. In this case the elective deep nodal region (N2) was not covered. There was concern about postoperative healing as there was major sternal reconstruction. There is a recurrence-free survival and overall survival (OS) benefit with PORT after resection of thymic carcinoma (2). However, the patient had only received 46 Gy when radiotherapy was discontinued as the patient was unable to tolerate further EBRT due to worsening dyspnea. A thoracentesis was performed to drain a pleural effusion; cytology was negative for malignant cells. Of note, the literature recommends a dose of 45–50 Gy after complete resection, and 50–54 Gy after R1 resection (2).
Disease progression
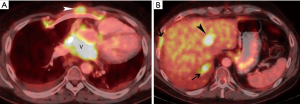
The patient’s dyspnea on exertion worsened 3 months after surgical resection resulting in a performance status of Eastern Cooperative Oncology Group (ECOG) 1. CT scan demonstrated local progression in the visceral mediastinum near the posterior pericardium (not shown). For patients who have received CAP as frontline therapy and have a good performance status, adequate organ function, and a long recurrence-free interval, options for combination therapy including carboplatin with paclitaxel, carboplatin or cisplatin with etoposide, and capecitabine with gemcitabine (2). Unfortunately, this patient’s disease recurred after a rather short disease-free interval. The patient received carboplatin with paclitaxel for three cycles with initial slight response but then progressed 4 months after starting chemotherapy, and nine months after initial surgery. FDG PET-CT imaging demonstrated progression of FDG-avid disease in the visceral mediastinum near the posterior pericardium and pleura, as well as uptake in a 2.2 cm hepatic lesion (Figure 4A,B). Recurrences of thymic epithelial tumors occur outside the mediastinum occur in more than 60% of cases (2). The 10-year OS for Masaoka-Koga stage IVB tumors is 53%, and the 10-year cumulative incidence of recurrence is 54% (2).
Questions for ITMIG tumor board
The patient is highly motivated and interested in available treatments, and the presenting physician is asking for the tumor board’s suggestions for further systemic therapy options.
ITMIG recommendations
The ITMIG medical oncologists agreed with the treating oncologist’s choice of second line chemotherapy. In non-resectable recurrences of thymic carcinoma several options for systemic therapy are available. Re-administration of a previously effective regimen should be considered if a considerable amount of time has elapsed after initial treatment (usually greater than 12 months). However, when considering re-administration of an anthracycline-containing regimen it should be noted that there is an increased risk of cardiac toxicity in patients who have received anthracyclines and mediastinal radiotherapy previously (2). One ITMIG medical oncologist noted this is an aggressive tumor and would change the systemic therapy regimen and consider sunitinib or a clinical trial. The second medical oncologist present agreed he would change the regimen and would be hesitant to prescribe another regimen of chemotherapy, given the limited effect of the first two lines, and instead would strongly recommend targeted therapy such as sunitinib or everolimus. The final group consensus is a new systemic regimen is recommended and the decision is largely empiric, although the presence of an actionable mutation such as a KIT mutation, although uncommon, might influence the choice of targeted therapy; meanwhile sunitinib was reported to produce significant disease control rate in the absence of such mutation through its anti-angiogenic mechanism of action.
In addition to the chemotherapy combinations mentioned above, drugs that can be used for single-agent therapy of recurrent thymic epithelial tumors include pemetrexed (greater degree of benefit in recurrent thymoma), paclitaxel, 5-fluorouracil or capecitabine, gemcitabine, etoposide, octreotide with or without prednisone (for tumors expression somatostatin receptors), sunitinib (for thymic carcinomas only) and everolimus (2). KIT sequencing is an option for refractory thymic carcinomas, particularly in the context of clinical trials (2). Occasionally, tumors might harbor KIT mutations and respond to imatinib, sorafenib, or sunitinib (2). Of note, clinical activity of sunitinib appears to be independent of the presence of a KIT mutation. However, the presence of a KIT mutation is necessary for the clinical activity of imatinib (2).
Of theoretical interest but not relevant for this patient as he experienced distant recurrence, there was discussion about the prescribed adjuvant EBRT. The planned dose of 50 Gy was agreed by the group to be acceptable, however one Radiation Oncologist present indicated they, as the local hospital did, would not cover the elective N2 region but would have more extensively covered the tumor bed plus margin as they personally have not experienced any healing difficulties in postoperative patients, which the presenter noted the local hospital was concerned about. An ITMIG working group and International Association for the Study of Lung Cancer (IASLC) collaboratively formed the Thymic Domain of the Staging and Prognostic Factors Committee (TD-SPFC) that defined the anterior nodal region N1 as bordered by the hyoid bone and diaphragm craniocaudally, the medial edge of the carotid sheaths and mediastinal pleura laterally, the sternum anteriorly, the pericardium and great vessels posteriorly in the middle, and extending to the level of the phrenic nerves posterolaterally (2). This region was in the field as this is the area of the tumor bed. A second ITMIG Radiation Oncologist would have covered the elective N2 region, defined as extending from the edges of the anterior region to the lateral border of the sternocleidomastoid muscle and the anterior edge of the vertebral column, and includes jugular, supraclavicular, aortopulmonary window, hilar, paratracheal, subcarinal, esophageal, internal mammary, and supradiaphragmatic nodes (2).
Case presentation 2
An 80-year-old woman, a very fit non-smoker, was being followed for a left lower lung (LLL) nodule demonstrating indolent growth as it had only grown a few millimetres over the preceding 5 years, and was suspected to be a typical carcinoid. A routine follow-up CT chest demonstrated a new station 6 enlarged node (Figure 5A) and the known LLL nodule (Figure 5B).
Imaging
She went on to have an FDG PET-CT scan that demonstrated intense uptake in the lymph node at station 6 (Figure 5C), very low FDG uptake in the LLL nodule that was lower than the mediastinal background activity (Figure 5D), and no other abnormalities were detected in the scan. The patient also had an MRI of her brain which was unremarkable.
Staging
The patient underwent video-assisted mediastinoscopic lymphadenectomy (VAMLA) which was negative for malignancy at sampled stations 2R, 4R, 4L, and 7.
Surgical management
The patient had a video-assisted thoracoscopic surgery (VATS) wedge resection of the LLL and dissection of nodal stations 5, 6, 8, 9L, and 11L. During surgery the surgeon noted the station 6 node, anterior to the left phrenic nerve, that appeared grossly malignant and adherent to the phrenic nerve. The surgeon felt this nodule could be completely resected with a good margin and this was attempted, however a small rim of the tumor estimated to be the ‘size of a grain of rice’ in the operative report was left behind on the phrenic nerve resulting in an R2 resection.
Pathology
The LLL nodule measured 9 mm. Surgical margins were negative consistent with R0 resection. Mitoses and necrosis were not present. Immunostains revealed that less than 1% of tumor cells were positive for Ki-67. The tumor cells expressed synaptophysin, CD56, TTF1, and chromogranin; they were negative for napsin, p40, and CK5/6. The station 6 node measured 1.1 cm. Unfortunately, microscopic images were not available at the time of the tumor board. Tumor cells in the station 6 node were positive for Ki-67 (10–20% of tumor cell nuclei staining), strongly positive for CD117 and CD5, positive for p40, CK5/6, and negative for synaptophysin, chromogranin, TTF1, and napsin. The stations 5, 8, 9L, and 11L nodes were negative for malignancy.
Diagnosis
The morphological and immunostaining results indicate the LLL nodule is consistent with typical carcinoid. The station 6 node was interpreted to be squamous cell carcinoma of thymic origin due to the immunostaining and no other primary site identified.
Questions for ITMIG tumor board
The presenter’s questions for the ITMIG tumor board were; would you re-resect the station 6 nodal area for an R0 resection, resulting in cutting the phrenic nerve; and would you resect the entire thymus even though it appears normal on CT and PDG PET-CT imaging?
ITMIG recommendations
This patient has TNM stage IVA (Tx N1 M0) thymic carcinoma and generally the treatment algorithm for a resectable stage IVA tumor is upfront surgery, PORT of 45–50 Gy with boost to areas of concern after an R0 resection and consider postoperative chemotherapy. Since this initially resectable tumor was not completely resected due to the portion of tumor remaining on the phrenic nerve and a thymectomy not being attempted, as initially this was being worked up as a lung primary, further management decisions are complex. If a stage IVA thymic carcinoma is initially evaluated to be unresectable the guidelines recommend the use of frontline, platinum-containing combination chemotherapy, then if the tumor becomes resectable surgery followed by PORT (2). At that point if the tumor remains unresectable or there is an R2 resection, definitive radiotherapy of 60 Gy or concurrent chemoradiotherapy can be considered (2). Several members noted they believed RT would be the best option in this case, though there is concern about reduced local control and would defer to the opinion of the surgeon at the local institution. Phrenic nerve preservation does not affect OS but increases the risk of local recurrence, and should be balanced with attainment of complete recurrence (2). Complete thymectomies that include removal of the tumor, residual thymus gland, and perithymic fat is preferred as local recurrences have been observed after partial thymectomies (2). An ITMIG surgical oncologist noted a second surgery would not be ideal due to the presence of adhesions and thought it would be difficult to achieve R0. In summary, the group came to the consensus that PORT is recommended by the ITMIG tumor board, and completion surgery to resect the thymus and cut the left phrenic nerve with attempted resection of the remaining station 6 node is not indicated in this case as it is unlikely to result in R0.
Case presentation 3
A 33-year-old man presented with symptoms compatible with myasthenia gravis and a mediastinal mass.
Imaging
Contrast enhanced chest CT imaging at presentation revealed a prevascular mass (Figure 6). Although there was no direct evidence of pericardial involvement, the fact that the tumor abutted a large surface of the pericardium and the border between them was indistinct was worrisome for pericardial involvement. There was no vascular invasion, pleural nodularity, nor distant metastatic disease.
Staging
Preoperative clinical staging showed at least T2 disease on CT imaging with suspected involvement of the pericardium (2).
Surgical management
This patient did not have a surgical biopsy prior to definitive treatment. Although theoretically a new mediastinal mass has a differential diagnosis, when the patient presents with myasthenia gravis and imaging is typical of an early-stage thymoma a biopsy is not needed prior to surgery because of the strong association of thymoma with myasthenia gravis (3). At surgery, the left phrenic nerve was encased by the tumor and the lingula was directly involved. The patient underwent definitive management with primary surgical resection of the mass, pericardium, lingula, and left phrenic nerve.
Pathology
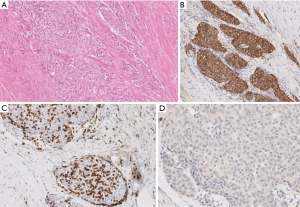
The surgical pathological specimen demonstrated features of a B3 thymoma, which was completely resected (R0) with negative margins. This tumor was staged as pT3 N0 M0 (Stage IIIA) disease (2). Microscopic images submitted for review were not sufficient to decisively establish the final diagnosis. Densely packed epithelioid cells with large nuclei and distinct nucleoli admixed with only singular lymphocytes may be appreciated (Figure 7A) and the neoplastic, epithelioid cells form nests surrounded by fibrotic stroma. Such morphology requires considering thymic carcinoma with desmoplastic stromal reaction, though the originally diagnosed B3 thymoma cannot be excluded. The immunohistochemical reaction anti-Tdt detecting immature T lymphocytes among neoplastic cells would enforce the thymoma diagnosis, though this test was not submitted for review. In Figure 7B the neoplastic cells expressed cytokeratin that confirmed their epithelial origin, and the cells were negative for CD5 (Figure 7C; positive reaction (brown) only expressed by lymphocytes) and CD117 (Figure 7D). Positive immunohistochemical reactions with CD5 and CD117 would advocate thymic carcinoma but negative reactions do not exclude it.
Diagnosis
The original histopathological diagnosis was B3 thymoma, and though the images submitted for review were not sufficient for confirmation of this entity, the morphological features support the diagnosis of a B3 thymoma with complete R0 resection. The patient did not receive any adjuvant treatment following initial surgical management.
Disease progression
Approximately 18 months after surgical resection, the patient developed dyspnea on exertion. The treating surgeon performed a plication of the diaphragm with a thoracotomy to help relieve his symptoms. At surgery he was found to have multiple significant pleural deposits. A contrast enhanced chest CT scan demonstrated new left pleural nodules measuring up to 3 cm in size and minute nodularity of the remaining pleura that may be additional metastatic pleural foci (Figure 8A). A biopsy of the pleural deposits confirmed metastatic B3 thymoma. The patient was subsequently treated with 4 cycles of CAP, followed by a left thoracotomy, pleurectomy, and diaphragmatic resection.
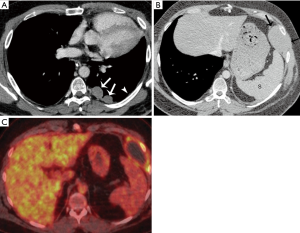
Eighteen months after the second surgical resection, a chest CT showed some thickening of the mediastinal pleura, which could be explained as postoperative changes. However, there was also a 5 cm × 3 cm pleural mass that developed at the insertion of the diaphragm to the chest wall. It bulged at the intercostal which suggested that the chest wall was likely involved (Figure 8B). A subsequent FDG PET-CT showed there was FDG uptake at the periphery of this mass (Figure 8C). The patient was treated with a chest wall resection, including resection of three anterior ribs, and residual phrenectomy with negative margins. The chest was thoroughly explored intraoperatively, and multiple intraoperative biopsies including that of the mediastinal pleura was obtained which were all negative for malignancy. An R0 resection was achieved. Final pathology demonstrated this was a recurrent B3 thymoma.
Questions for ITMIG tumor board
The presenter’s questions for the ITMIG tumor board were: would you have offered adjuvant chemotherapy or adjuvant radiation therapy after the first surgical resection, and at this time would you offer radiation therapy for the chest wall metastasis?
ITMIG recommendations
This patient initially had a TNM stage IIIA (pT3 N0 M0) B3 thymoma which was completely resected with negative margins (R0). In patients who are operative candidates the definitive management of thymomas includes complete resection of the tumor and total thymectomy. R0 resection is the most important prognostic factor (2). The 5-year OS for patients with stages III or IV thymoma with complete resection approaches 90% (4). Consensus guidelines suggest that following complete resection there is no role for adjuvant chemotherapy (5). There was agreement among the ITMIG medical oncologists that no adjuvant chemotherapy was required after the initial resection. Furthermore, consensus guidelines suggest there is no role for elective nodal radiation in node negative patients as thymomas have a low propensity for nodal spread (1). However, in patients with stage III disease PORT can be appropriate as these patients are at increased risk of local recurrence (6-8). The adjuvant dose for radiation therapy in patients with close or clear margin resections is between 45–50 Gy with conventional fractionation (1). Although PORT might not be beneficial in this patient considering the location of the recurrence was not within the tumor bed, PORT should be offered to patients with stage III B3 thymoma.
In patients who have recurrent disease the consensus guidelines suggest treatment with chemotherapy and/or with surgery for isolated metastases. The preferred chemotherapy regimen in thymoma is CAP (1). Carboplatin with paclitaxel is a non-anthracycline-containing option for thymoma and thymic carcinoma (1). Radiation therapy can also be given in the recurrent setting and stereotactic body radiation therapy (SBRT) may be considered for isolated metastatic disease (1). The ITMIG participants agreed with the management of recurrent pleural disease with chemotherapy followed by definitive surgical management in this setting. One of the members of the ITMIG suggested surgical resection of the pleural metastases if it is isolated, and intra-pleural chemotherapy for extensive disease.
Regarding the second recurrence to the chest wall, the group consensus was for surgical resection of the chest wall mass given the patient’s young age. An ITMIG radiation oncologist spoke about agreeing with the decision to surgically resect the chest wall recurrence but warned that more metastatic deposits may develop in the future and SBRT may be an option. One of the thoracic surgeons spoke of a similar case, in which the patient had surgery but later developed multiple diaphragmatic nodules. Therefore, this individual proposed a more aggressive approach with complete removal of the diaphragm and a pre-operative MRI.
In summary, the groups consensus was there was no role for adjuvant chemotherapy after the original resection, while adjuvant RT could be considered. There was agreement with using chemotherapy followed by surgical resection in the setting of recurrent pleural metastatic disease. Lastly, in the setting of a second recurrence of the chest wall, the group agreed that surgical resection of an isolated metastasis with a partial pleurectomy was reasonable and concluded that no further chemotherapy is indicated, and the patient may simply be observed.
Acknowledgments
We would like to thank all the ITMIG members and the colleagues present for the meeting who made the discussion and this publication possible, including the administrative support of P. Bruce. Additionally, we would like to thank the pathologists from the consulting institutions who made this publication possible by allowing us to publish their images.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2020.01.01). ACR serves as an unpaid Associate Editor of Mediastinum from May 2017 to Apr 2019 and from Jul 2019 to Jun 2021. MM and CBF serves as an unpaid editorial board member of Mediastinum from May 2017 to Apr 2019 and Jul 2019 - Jun 2021. EMM reports honorarium for lecture from Bristoll-Meyers Squibb, Boehringer Ingelheim, and Merck Sharp and Dohme, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Informed consent was obtained from the patients for publication of this article and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ettinger DS, Wood DE, Aisner DL, et al. Thymomas and thymic carcinomas [v2.2019]. In: National Comprehensive Cancer Network. 2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/thymic.pdf. Accessed May 2019.
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG thymic epithelial tumors staging project: Proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S65-72. [Crossref] [PubMed]
- Detterbeck FC, Parsons AM. Management of stage I and II thymoma. Thorac Surg Clin 2011;21:59-67. vi-vii. [Crossref] [PubMed]
- Zhao Y, Shi J, Fan L, et al. Surgical treatment of thymoma: An 11-year experience with 761 patients. Eur J Cardiothorac Surg 2016;49:1144-9. [Crossref] [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84. [Crossref] [PubMed]
- Jackson MW, Palma DA, Camidge DR, et al. The impact of postoperative radiotherapy for thymoma and thymic carcinoma. J Thorac Oncol 2017;12:734-44. [Crossref] [PubMed]
- Lim YJ, Kim E, Kim HJ, et al. Survival impact of adjuvant radiation therapy in Masaoka stage II to IV thymomas: A systematic review and meta-analysis. Int J Radiat Oncol Biol Phys 2016;94:1129-36. [Crossref] [PubMed]
- Rimner A, Yao X, Huang J, et al. Postoperative radiation therapy is associated with longer overall survival in completely resected stage II and III thymoma: An analysis of the International Thymic Malignancies Interest Group retrospective database. J Thorac Oncol 2016;11:1785-92. [Crossref] [PubMed]
Cite this article as: Sigurdson S, Moideen N, Marom EM, Szolkowska M, Roden AC, Rajan A, Girard N, Marino M, Noh JM, Kirk A, Detterbeck FC, Falkson CB. Case presentations and recommendations from the 2018 ITMIG Annual Meeting. Mediastinum 2020;4:7.


