Video assisted thoracoscopic surgery in paediatric mediastinal tumors
Introduction
Video assisted thoracoscopic surgery (VATS) was performed in children as early as 1970’s for obtaining biopsy of mostly infectious lesions (1). Initially benign conditions like cyst excision, sequestration resection, lung biopsies and lobectomies were tried with VATS (2). With the technological advances in visualization systems and instrumentation, the range of VATS application has gained momentum from biopsy to advanced thoracic surgeries competing with open techniques. One of the major reasons for acceptance of VATS in thoracic community was the morbidities associated with thoracotomies. Around 10% to 15% of paediatric patients undergoing major thoracotomies will have some form of muscle girdle weakness, chest wall deformity or scoliosis (3). In contrast, most procedures by VATS can be done using three or four 3–5 mm ports and a small working incision (if required), thereby significantly decreasing chest wall related morbidities and a better cosmetic outcome (4). Surgeons had explored and established feasibility of VATS in all areas of thorax including patent ductus arteriosus repair to complex trachea-esophageal fistulas with results equivalent to open surgery (5,6). However, the surgery for solid mediastinal tumors are based on strict oncologic principles rather than feasibility. So an experience of both oncologic surgery along with VATS is necessary.
Guidelines for minimally invasive oncologic surgery in children are not standardized and no randomised trial has addressed these issues yet (7). This is reflected by the limited application of VATS seen in malignant mediastinal tumors in children. Even large institutions included only a small volume of cases in their studies, mostly 10–30 patients (8). Contrary to this, VATS has been used extensively for mediastinal tumors in adults including single port VATS (9,10). The most common reason likely is its extreme rarity. An analysis of the SEER database of New Mexico over a 22-year period revealed that paediatric malignant mediastinal tumors were seen in one out of every 5,012 malignancies in the population (11). This was 20 times less common than what was observed among adults. Secondly, most mediastinal tumors (up to 50%) in children are malignant unlike 10–15% in adults (12). Larger size of these malignant mediastinal tumors with the smaller thoracic cage makes retrieval difficult, anyway requiring a larger incision, thus defeating the advantage of VATS. Lastly, there has been a fear of achieving an incomplete tumor free margin with VATS due to the larger tumor size. Hence there is an ongoing debate on the acceptance of this minimally invasive procedure in children.
This review aims to analyse these controversies for application of VATS in mediastinal tumors in children and would try to find out areas of improvement attempted by various centres.
Outline of nomenclatures
VATS types
As VATS is mostly used for lung surgeries, the nomenclatures were defined in the context of them. Two types of VATS approaches were generally described and validated (13,14). Complete VATS (C-VATS) which consist of a utility incision along with the thoracoscopic ports. The utility incision is a small thoracotomy incision via which thoracoscopic instruments were introduced for retraction only. Second type is the assisted VATS (A-VATS) where the utility incision is extended as required to actually visualise and dissect through it rather than solely relying on the thoracoscopic ports.
Paediatric age definitions in literature
The upper age limit of inclusion or exclusion to VATS has been quite variable from institution to institutions (15). A wide range is observed in various articles of paediatric mediastinal tumors starting from 14 years to the oldest case even 19 years (16). This is an area of contention because the adult chest wall anatomy is nearly reached by the end of first few years of life (discussed below in detail).
Growing child issues for the VATS patient
Does chest wall anatomy in children differ from adults?
Openshaw and colleagues identified two important developmental changes in rib cage geometry as the infant grows up (17). First is the horizontal lie of the ribs along with higher position of sternal clavicular heads and diaphragmatic domes. Second is the cross-sectional changes, where the round infantile shape changes to oval adult shape due to rib growth at costochondral junctions and posterior rib angles. The left diaphragmatic dome descends from 8th thoracic vertebra (T8) level to T11. The right dome remains half vertebral space higher than the left. They concluded that much of this adult shape is attained by the age of 2–3 years. This is important to consider as an oval and expansile chest wall offers better visibility and work-space for VATS. Also, the sternal angle is not prominent in these first few years which is a commonly used landmark by thoracic surgeons for identifying the correct intercostal space (ICS). Other age-related changes in vertebral column and sternum seem less likely to affect VATS.
Managing VATS with developmental defects in chest wall anatomy?
VATS surgeons always anticipate the possibility for conversion to open procedure when they plan for port placement. This planning can be affected by abnormalities in growth and development of the child and needs a mention. Defects of rib cage frequently encountered in thoracic surgery are supernumerary ribs in cervical or lumbar areas, fused and bifid ribs (18). Defects with absence or rudimentary ribs (for instance Poland syndrome) are less common but do occur. With growth, these chest wall defects become more pronounced but simultaneously more stable to deal with. Sternal defects like pectus excavatum may reduce the working space for surgery. A bifid sternum on the other hand is rare and do not pose a problem for VATS. Although there were no reports with mediastinal tumors, lung surgeries in children had been successfully performed along with chest wall deformities in the same setting by VATS with minimal morbidities (19).
Is VATS safe in malignant mediastinal tumors?
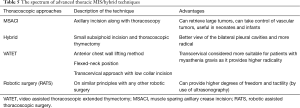
With decreasing age, the safety concerns for a newer procedure increase. A 10-year experience from Rothenberg and colleagues showed that these minimally invasive procedures were safe, effective and associated with same benefits in infants as older patients (20). Table 1 describes the usual post-operative complications seen in various studies (8,21,22).
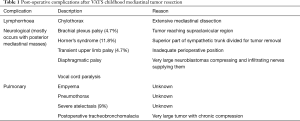
Full table
Anaesthetic and perioperative concerns
Exposure is an essential part of minimally invasive thoracic surgery and a good ipsilateral lung deflation is always helpful. To achieve this, the anaesthetists require a thin scope and small lumen endotracheal tubes. Bronchoscopes need to be <90% of the inner diameter of the endotracheal tube to negotiate through the tube (23). But in order to continue to ventilate while still inside, the outer diameter of the bronchoscope should occupy <50% of the cross-sectional area of the inner diameter of the endotracheal tube. A double lumen endotracheal tube provides the most optimal lung isolation (24). However, if a single lumen tube (SLT) is only available then a bronchial blocker can be used to isolate the desired lung. In situations where thin bronchoscope is not available, an ordinary cuffed SLT can be introduced into the non-operated side and position can be checked clinically or radiologically (25). A bronchial blocker can get dislodged during patient positioning or any time during surgery making things difficult for the surgeon. Disadvantages of a SLT include an inability to adequately deflate or suction the airway on the operating side. Other methods like use of uninvent tube, two SLTs and Fogarty embolectomy balloon catheters have also been described (26).
Do children tolerate positive pressure insufflation of thorax like adults?
If for any reason lung deflation is inadequate for exposure then positive pressure insufflation of the thoracic cavity is an option. Positive insufflation (capnothorax) is associated with various risks. Animal studies have shown that capnothorax with pressures >5 mmHg causes decrease in cardiac index, mean arterial pressure, left ventricular stroke volume while an increase in pulmonary artery and central venous pressures (27). With higher pressures (10–15 mmHg) even myocardial infarction was also reported in these animal models (28). Studies in adult human had shown that although patients can have the aforementioned adverse effects but clinical effects were insignificant when carbon dioxide (CO2) insufflation pressures were kept below 10 mmHg (29). Because of lower tolerance than adults, a low flow positive pressure CO2 insufflation (1 L/min) at low pressure (4–6 mmHg) is used in smaller children (30). Besides these as children are not small adults, there are other aspects of physiology associated with capnothorax (31). CO2 is a cool and dry gas which can cause hypothermia in very small children. Increasing the flow of the gas to compensate for usual trocar site leaks can also add to this problem. This increases the intrathoracic pressure which can result in sudden mediastinal shift and cardiovascular collapse (32,33). Rarely, CO2 gas embolism can occur (34). An experienced team following safe anaesthetic practices needs to understand these minimally invasive issues and manage accordingly (35).
Can VATS compete technically with open surgery for mediastinal tumors?—surgical aspects
Can a complete resection be achieved with equal success rates like open surgeries?
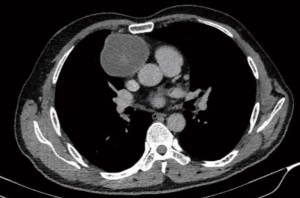
Early studies with VATS thymectomies for myasthenia gravis (MG) in children has established its ability to achieve a complete resection and benefit similar to adult series (36,37). DeCou et al. reported resection in 5 children with neuroblastomas via thoracoscopy and achieved a complete resection with good short term survival (38). However, they had tumor spillage in a couple of cases. All tumors were stage 1 and ≤6 cm size. So the feasibility of VATS in well encapsulated tumors (Figure 1) is well established but those with large tumors with higher stages remains a concern.
Is there a recommended size?
A larger size of tumor needs more manipulation and hence the risk of tumor rupture and seeding of the pleural cavity. While there were no recommendations to a specific size but most studies done for adult VATS thymectomy consider 5 cm as the maximum feasible diameter for safe resection (39). Due to extensive variation of chest size in children, it is the ratio of tumor to the ipsilateral thoracic cavity which seems a more logical approach to decide rather than the absolute tumor size.
Concerns about margin positivity in malignant tumors and gross subtotal resections?
The need for a margin negative resection has been one of the key oncologic principles. Most of these issues came up after the introduction of VATS thymectomies and use of heterogeneous terms in literature, “complete”, “extended” or “maximal” thymectomy (40). Opponents of a complete resection argue that in early stage non-invasive (stages I and II) thymoma without MG a subtotal thymectomy or a thymectomy done through VATS can provide acceptable results like thymectomy via an open approach (41,42). They had also reported that none of their patients developed MG after a follow up period of 57 months and there was no significant difference in recurrence rates. Hence for early non-MG thymomas, a subtotal thymectomy is considered justified although long term studies are necessary in this regard (43). Still many surgeons continue to perform complete resection of thymoma expecting decreased risk of MG and higher recurrence free and overall survival (44). However MG can still develop after a complete resection (45).
Paediatric mediastinal neurogenic tumors of thorax are uncommon and present sometimes uniquely with intraspinal extension posing a challenge to VATS. Fraga and colleagues reported that a gross clear resection can be achieved by VATS and results comparable with thoracotomy (21). For International Neuroblastoma Staging System (INSS) stage I neuroblastomas, surgery alone is sufficient treatment even in the presence of a positive margin or unfavourable biologic prognostic factors (46).
What is the evidence for lymph node dissection today and is it achievable by VATS?
Few studies have addressed the issue of lymph node dissection and most were in adults. Based on a study of 37 patients of thymic carcinoma, Park et al. recommended extensive lymph node dissection (minimum 10 nodes) to predict prognosis accurately (47). The SEER database analysis of 442 adult patients with thymoma resection showed that the incidence of lymph node metastasis is 13.3% (48). The study concluded that sampling of at least the anterior mediastinal nodes as per the IASLC/ITMIC proposal and AJCC 8th edition changes should be done in all cases as nodal metastasis has a high prognostic value (49). But 55% of the patients in this study were Masoka-Koga stages III and IV and only those cases were included where at least one lymph node was dissected along with the specimen. Contrary to this, Kondo et al. reported the incidence on lymph node metastases in more than 1,000 patients of thymoma as 1.8% with almost 25% patients in stages III and IV (50). They reported a higher incidence of lymph node metastases in thymic carcinoma (27%) and thymic carcinoid (28%) among the 186 and 41 patients respectively. Hence, there is a wide variation in incidence of lymph node metastasis.
Thymoma is considered as a relatively indolent disease. In a 20-year retrospective analysis by Okereke showed that only 4.3% patients had died of thymoma while most died of non-thymic causes thereby overestimating the need for a lymph node dissection (51). The overall role of lymph node dissection in thymoma is controversial with lymph node dissection described only for prognostic importance without any survival benefit. Considering all these evidences, a dissection or sampling of anterior mediastinal lymph nodes in thymic tumors (especially the higher stage thymomas, thymic carcinoma and carcinoids) is required.
The adequacy of lymph node dissection by VATS compared to open thoracotomy has been a matter of debate with most data derived from observations among adult patients. The ChART prospective multicentre analysis by the Fang group reported that when compared to open surgery, patients undergoing VATS had a higher number of mean lymph nodes (5.31 vs. 4.46), lymph node stations (3.30 vs. 2.84) and N2 dissections (1.57 vs. 1.45) respectively (52). The role of lymph node dissection in other paediatric mediastinal tumors like neuroblastomas or germ cell tumors is not clear.
Conversion rates from VATS to open—is it different from adults?
It is hard to find conversion rates specific to malignant mediastinal tumor as most of the studies report the experience of VATS in childhood thoracic tumors as a whole where lung surgeries also get included. The children’s cancer group reported a 9.5% conversion rate of VATS procedures for tissue biopsy purposes (53). A 5-year retrospective review from 7 Italian SIVI centres operating for 5 thoracoscopic mediastinal mass excisions and 14 mediastinal biopsies showed a 100% success rate without any conversion to open (54). Considering specifically for thoracoscopic mass excision, the German group could complete VATS in 7 out of 11 attempted thoracoscopic cases, having a conversion rate of 36.4% (55). Only one among the 14 attempted biopsy procedures was converted.
Friedant et al. conducted a meta-analysis of adult thymectomy patients comparing the feasibility of VATS with open procedures (56). Although conversion rates were not the primary aim of the study but they found that out of 1,355 cases posted for VATS, 32 were converted to open procedures accounting for a conversion rate of 2.36%. All of these indicate a higher conversion rate among children although a good success rate could be achieved in the successful cases. Some of the factors mentioned in literature to make a wise decision for conversion to an open procedure have been discussed in Table 2.

Full table
Risk of port site recurrences?
Tumor recurrences at port site after VATS paediatric mediastinal tumor resection is a rare phenomenon (57). All reported recurrences occurred after minimally invasive thoracoscopic resection were for pulmonary metastases or carcinoma and mostly in adults with incidences of around 0.26% (58-61). In paediatric VATS, the only case reported was a 18-year-old female who presented with port site metastases within 4 months of pulmonary metastasectomy for osteosarcoma (62). Still, the recommended practice is to retrieve the surgical specimen using a plastic bag (63). Nonetheless, a case report for port site recurrence due to benign tumor has also been reported (64).
Advantages of VATS
Perioperative pain control
Centres performing VATS thymectomies report this procedure as less traumatic, decreased postoperative morbidities (especially pulmonary complications and pain), lower intraoperative blood loss, shorter ICU and hospital stay with no significant differences in operating time (65,66). Patients with MG have an increased risk of respiratory muscle weakness which can further add to decreased postoperative respiratory efforts and thereby increasing chances of pulmonary complications. Hence minimally invasive procedure can have an edge over open surgery in this regard. Further, it is the spreading of ribs which is the main cause of wound discomfort rather than muscle cutting incision as previously thought (67). Table 3 provides a brief description of the various anaesthetic and perioperative pain control methods used in paediatric VATS (68-70).

Full table
Is there a survival benefit?
Retrospective studies involving VATS thymectomy in adults have argued the possibility of a 5-year overall survival benefit without any difference in recurrence free survival (66). They included stage I or II thymomas where recurrences and survival need long term follow up. Such a benefit has not been described in paediatric literature. Decreased operating time, faster recovery and cost was observed for benign paediatric thoracoscopic procedures (71,72). Survival assessments need long term studies with larger number of patients which is only possible in adults where we have sizeable data. Hence most conclusions were derived from studies done in adult patients. A systematic review of 2,068 adult patients of thymectomy showed no difference in thymoma recurrence, MG complete remission and 5-year survival between VATS and open procedures (73). Fraga et al. observed no recurrence in mediastinal neurogenic tumors in children at a median follow-up of 16 months (22). Although a long-term benefit has not been reported, the avoidance of thoracotomy complications and better visualisation were described as the main advantages of paediatric VATS.
Early start of adjuvant treatment?
Due to a rapid post-operative recovery there is decrease in hospital stay which helps in starting adjuvant treatment without delay (74). Although it seems reasonable to believe that this can be a reason for survival benefit observed by some with VATS but the authors did not comment on any such an association in their study.
Patient selection, technical issues and tips
There is no absolute contraindication to VATS in paediatric mediastinal tumors. However a proper patient selection is the key (Table 4) (75,76). Besides complete excision, VATS can be used as a diagnostic tool for biopsy of mediastinal tumors or lymph nodes in patients where an image guided tissue diagnosis is inadequate and radiological features suspect haematological malignancies, benign solid tumors or non-neoplastic conditions (77).
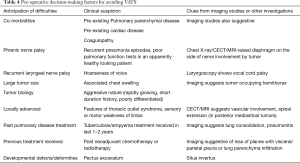
Full table
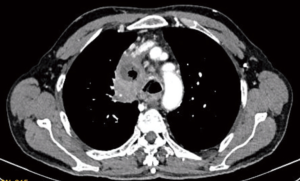
Presence of features of locally advanced disease (Figure 2) makes decision easier but this is not the case always. VATS should be seen only as a minimally invasive tool for mediastinal tumors resection and the limitations of this approach should not be considered as its disadvantages. Most of its disadvantages like high infrastructure costs and limitation of tactile sensation were similar to any other minimally invasive procedure. However, in infants and smaller children paediatric VATS demands very small calibre instruments which may not be easily available. Due to lower incidence of cases and technical and structural problems there is a slower progress in achieving experience in VATS even among purely paediatric centres (78). This leads to scarcity of adequate training facilities when compared with adults.
From the experiences of various authors, few salient tips can be useful for the beginner VATS surgeons. Adequate planning should involve correct patient positioning and port placement which is the first step towards a successful resection. One of the largest series by Partrick et al. suggested that a prone position is better for posterior mediastinal tumor resection as it allows the retraction of collapsed lung by gravity (79). Frequently the 4th or 5th ICS is used for placement of camera port but for older children 7th or 8th ICS is more suitable (80). As the thoracic cage is variable across different ages in children, port placement has to be planned both according to disease site and specific individual. The entry of the first port (camera) needs caution as introducing through areas of lung adhesion can cause an inadvertent lung parenchymal injury. A clue to this may sometimes be available in the preoperative imaging. Rarely diaphragmatic injury can occur after introduction of the first trocar if it is at 7th ICS or lower (81). In cases of bleeding after biopsy of a friable mediastinal mass a “figure of 8” suture can be useful for haemostasis (82). Finally, specimen should always be retrieved in a plastic bag as already discussed and if it is not available then at least a wound protector should be in situ (83).
Does a learning curve in VATS exist?
Adult studies have shown that VATS is associated with a well described learning curve (84). The existence of a similar learning curve is seen in paediatric patients (78,85). As the number of cases are fewer in case of children so gathering experience takes longer and so is the training. With appropriate training program as seen with adult patients, the peak of this learning curve can be reached after 50 cases (86).
Specific oncologic issues
Does post neo-adjuvant setting make VATS difficult?
Although adhesions are expected after neo-adjuvant therapy but VATS can be performed successfully in these setting and conversion to open should be considered according to the situation (16).
Role of a chest tube?
The need for a chest tube after thoracoscopic procedures in children has been questioned by few authors with the reason that it is more painful post-operatively and keeping the tube does not have any additional benefits (87). Many consider an early removal of chest tube within 24 hours of surgery (79).
Is data collection and audit necessary?
It is recommended that all centres performing VATS should collect data and audit their procedures for perioperative outcomes including long term morbidity and mortality (88). The ITMIG group has prescribed proformas for recording intraoperative notes and data collection for minimally invasive surgery in thymoma (83). Only audit of a well collected data can give a useful analysis regarding the performance of the centre and identify areas of improvement.
VATS: a combined decision
With understanding of the various aspects of VATS in children, the decision to consider for either minimally invasive or thoracotomy should be made jointly by a multidisciplinary team involving the paediatrician and thoracic surgeon (16).
Current direction and future trends
VATS in children has been an area of interest among surgeons with the growing advances in technology. It is a well-established and standard technique for childhood mediastinal tumors. Refinement of this technique and technological advances will lead to better ways of achieving results. A variety of modifications and hybrid methods have been attempted by surgeons with reported benefits (Table 5) (85,89-93). Robotic VATS is another such addition to the armamentarium of the surgeon with better freedom of movement, vision and hence handling difficult cases. While these systems entail a higher infrastructure and investment cost but in the long term may prove promising just like other fields of surgery. Moreover, the upcoming of intraoperative ultrasonography which is already inbuilt in the newer da Vinci robotic systems can decrease the guess work and wait time for decision to convert to a thoracotomy.
Full table
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor Deepali Jain for the series “Pediatric Mediastinal Tumors” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2019.09.04). The series “Pediatric Mediastinal Tumors” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rodgers BM, Talbert JL. Thoracoscopy for diagnosis of intrathoracic lesions in children. J Pediatr Surg 1976;11:703-8. [Crossref] [PubMed]
- Cano I, Antón-Pacheco JL, García A, et al. Video-assisted thoracoscopic lobectomy in infants. Eur J Cardiothorac Surg 2006;29:997-1000. [Crossref] [PubMed]
- Rothenberg SS. Thoracoscopy in infants and children: the state of the art. J Pediatr Surg 2005;40:303-6. [Crossref] [PubMed]
- Lawal TA, Gosemann JH, Kuebler JF, et al. Thoracoscopy versus thoracotomy improves midterm musculoskeletal status and cosmesis in infants and children. Ann Thorac Surg 2009;87:224-8. [Crossref] [PubMed]
- Nezafati MH, Soltani G, Mottaghi H, et al. Video-assisted thoracoscopic patent ductus arteriosus closure in 2,000 patients. Asian Cardiovasc Thorac Ann 2011;19:393-8. [Crossref] [PubMed]
- Rothenberg SS. Thoracoscopic repair of esophageal atresia and tracheo-esophageal fistula in neonates: evolution of a technique. J Laparoendosc Adv Surg Tech A 2012;22:195-9. [Crossref] [PubMed]
- van Dalen EC, de Lijster MS, Leijssen LG, et al. Minimally invasive surgery versus open surgery for the treatment of solid abdominal and thoracic neoplasms in children. Cochrane Database Syst Rev 2015;1:CD008403 [PubMed]
- Sato T, Kazama T, Fukuzawa T, et al. Mediastinal tumor resection via open or video-assisted surgery in 31 pediatric cases: Experiences at a single institution. J Pediatr Surg 2016;51:530-3. [Crossref] [PubMed]
- Wu CY, Heish MJ, Wu CF. Single port VATS mediastinal tumor resection: Taiwan experience. Ann Cardiothorac Surg 2016;5:107-11. [Crossref] [PubMed]
- Rowse PG, Roden AC, Corl FM, et al. Minimally invasive thymectomy: the Mayo Clinic experience. Ann Cardiothorac Surg 2015;4:519-26. [PubMed]
- Temes R, Allen N, Chavez T, et al. Primary mediastinal malignancies in children: report of 22 patients and comparison to 197 adults. Oncologist 2000;5:179-84. [Crossref] [PubMed]
- Davis RD, Oldham HN, Sabiston DC. Primary cysts and neoplasms of the mediastinum: recent changes in clinical presentation, methods of diagnosis, management, and results. Ann Thorac Surg 1987;44:229-37. [Crossref] [PubMed]
- Shigemura N, Akashi A, Nakagiri T, et al. Complete versus assisted thoracoscopic approach: a prospective randomized trial comparing a variety of video-assisted thoracoscopic lobectomy techniques. Surg Endosc 2004;18:1492-7. [Crossref] [PubMed]
- Shigemura N, Akashi A, Funaki S, et al. Long-term outcomes after a variety of video-assisted thoracoscopic lobectomy approaches for clinical stage IA lung cancer: a multi-institutional study. J. Thorac Cardiovasc Surg 2006;132:507-12. [Crossref] [PubMed]
- Train AT, Harmon CM, Rothstein DH. Influence of hospital-level practice patterns on variation in the application of minimally invasive surgery in United States pediatric patients. J Pediatr Surg 2017;52:1674-80. [Crossref] [PubMed]
- Koizumi K, Haraguchi S, Hirata T, et al. Thoracoscopic surgery in children. J Nippon Med Sch 2005;72:34-42. [Crossref] [PubMed]
- Openshaw P, Edwards S, Helms P. Changes in rib cage geometry during childhood. Thorax 1984;39:624-7. [Crossref] [PubMed]
- Ehrenhaft JL, Rossi NP, Lawrence MS. Developmental chest wall defects. Ann Thorac Surg 1966;2:384-98. [Crossref] [PubMed]
- Bilgi Z, Ermerak NO, Bostancı K, et al. Feasibility and Complications in Concomitant Lung Resection With Minimally Invasive Repair of Pectus Excavatum. Ann Thorac Surg 2015;100:707-9. [Crossref] [PubMed]
- Rothenberg SS, Chang JHT, Bealer JF. Minimally Invasive Surgery in Neonates: Ten Years’ Experience. Pediatr Endosurgery Innov Tech 2004;8:89-94. [Crossref]
- Fraga JC, Aydogdu B, Aufieri R, et al. Surgical treatment for pediatric mediastinal neurogenic tumors. Ann Thorac Surg 2010;90:413-8. [Crossref] [PubMed]
- Fraga JC, Rothenberg S, Kiely E, et al. Video-assisted thoracic surgery resection for pediatric mediastinal neurogenic tumors. J Pediatr Surg 2012;47:1349-53. [Crossref] [PubMed]
- Letal M, Theam M. Paediatric lung isolation. BJA Educ 2016;17:57-62. [Crossref]
- Brodsky JB. Lung separation and the difficult airway. Br J Anaesth 2009;103:i66-75. [Crossref] [PubMed]
- Baraka A, Slim M, Dajani A, et al. One-lung ventilation of children during surgical excision of hydatid cysts of the lung. Br J Anaesth 1982;54:523-8. [Crossref] [PubMed]
- Fabila TS, Menghraj SJ. One lung ventilation strategies for infants and children undergoing video assisted thoracoscopic surgery. Indian J Anaesth 2013;57:339-44. [Crossref] [PubMed]
- Hill RC, Jones DR, Vance RA, et al. Selective lung ventilation during thoracoscopy: effects of insufflation on hemodynamics. Ann Thorac Surg 1996;61:945-8. [Crossref] [PubMed]
- Sato M, Muraji T, Asai T, et al. Hemodynamic Effects of Carbon Dioxide Insufflation of the Thoracic Cavity During Thoracoscopic Surgery. Pediatr Endosurgery Innov Tech 2002;6:185-9. [Crossref]
- Wolfer RS, Krasna MJ, Hasnain JU, et al. Hemodynamic effects of carbon dioxide insufflation during thoracoscopy. Ann Thorac Surg 1994;58:404-7; discussion 407-8. [Crossref] [PubMed]
- Dave N, Fernandes S. Anaesthetic implications of paediatric thoracoscopy. J Minim Access Surg 2005;1:8-14. [Crossref] [PubMed]
- Mitul AR, Sarin YK. Minimal Access Surgery in Neonates. J Neonatal Surg 2017;6:59. [Crossref] [PubMed]
- Conacher ID. Anaesthesia for thoracoscopic surgery. Best Pract Res Clin Anaesthesiol 2002;16:53-62. [Crossref] [PubMed]
- Haynes SR, Bonner S. Review article: anaesthesia for thoracic surgery in children. Paediatr Anaesth 2000;10:237-51. [Crossref] [PubMed]
- Tobias JD. Anaesthetic implications of thoracoscopic surgery in children. Paediatr Anaesth 1999;9:103-10. [Crossref] [PubMed]
- Kumar K, Basker S, Jeslin L, et al. Anaesthesia for Pediatric Video Assisted Thoracoscopic Surgery. J Anaesthesiol Clin Pharmacol 2011;27:12-6. [PubMed]
- Parikh K, Vaidya A, Jain R. Preliminary results of VATS thymectomy for pediatric myasthenia gravis. Pediatr Surg Int 2011;27:595-8. [Crossref] [PubMed]
- Skelly CL, Jackson CCA, Wu Y, et al. Thoracoscopic thymectomy in children with myasthenia gravis. Am Surg 2003;69:1087-9. [PubMed]
- DeCou JM, Schlatter MG, Mitchell DS, et al. Primary thoracoscopic gross total resection of neuroblastoma. J Laparoendosc Adv Surg Tech A 2005;15:470-3. [Crossref] [PubMed]
- Takeo S, Tsukamoto S, Kawano D, et al. Outcome of an original video-assisted thoracoscopic extended thymectomy for thymoma. Ann Thorac Surg 2011;92:2000-5. [Crossref] [PubMed]
- Toker A. Standardized definitions and policies of minimally invasive thymoma resection. Ann Cardiothorac Surg 2015;4:535-9. [PubMed]
- Sakamaki Y, Kido T, Yasukawa M. Alternative choices of total and partial thymectomy in video-assisted resection of noninvasive thymomas. Surg Endosc 2008;22:1272-7. [Crossref] [PubMed]
- Odaka M, Akiba T, Yabe M, et al. Unilateral thoracoscopic subtotal thymectomy for the treatment of stage I and II thymoma. Eur J Cardiothorac Surg 2010;37:824-6. [Crossref] [PubMed]
- Tseng YC, Hsieh CC, Huang HY, et al. Is thymectomy necessary in nonmyasthenic patients with early thymoma? J Thorac Oncol 2013;8:952-8. [Crossref] [PubMed]
- Regnard JF, Magdeleinat P, Dromer C, et al. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg 1996;112:376-84. [Crossref] [PubMed]
- Kondo K, Monden Y. Myasthenia gravis appearing after thymectomy for thymoma. Eur J Cardiothorac Surg 2005;28:22-5. [Crossref] [PubMed]
- Kushner BH, Cheung NK, LaQuaglia MP, et al. International neuroblastoma staging system stage 1 neuroblastoma: a prospective study and literature review. J Clin Oncol 1996;14:2174-80. [Crossref] [PubMed]
- Park IK, Kim YT, Jeon JH, et al. Importance of lymph node dissection in thymic carcinoma. Ann Thorac Surg 2013;96:1025-32; discussion 1032. [Crossref] [PubMed]
- Weksler B, Pennathur A, Sullivan JL, et al. Resection of thymoma should include nodal sampling. J Thorac Cardiovasc Surg 2015;149:737-42. [Crossref] [PubMed]
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S65-72. [Crossref] [PubMed]
- Kondo K, Monden Y. Lymphogenous and hematogenous metastasis of thymic epithelial tumors. Ann Thorac Surg 2003;76:1859-64; discussion 1864-5.
- Okereke IC, Kesler KA, Morad MH, et al. Prognostic indicators after surgery for thymoma. Ann Thorac Surg 2010;89:1071-7; discussion 1077-9. [Crossref] [PubMed]
- Fang W, Wang Y, Pang L, et al. Lymph node metastasis in thymic malignancies: A Chinese multicenter prospective observational study. J Thorac Cardiovasc Surg 2018;156:824-33.e1. [Crossref] [PubMed]
- Holcomb GW, Tomita SS, Haase GM, et al. Minimally invasive surgery in children with cancer. Cancer 1995;76:121-8. [Crossref] [PubMed]
- Esposito C, Lima M, Mattioli G, et al. Thoracoscopic surgery in the management of pediatric malignancies: a multicentric survey of the Italian Society of Videosurgery in Infancy. Surg Endosc 2007;21:1772-5. [Crossref] [PubMed]
- Metzelder ML, Kuebler JF, Shimotakahara A, et al. Role of diagnostic and ablative minimally invasive surgery for pediatric malignancies. Cancer 2007;109:2343-8. [Crossref] [PubMed]
- Friedant AJ, Handorf EA, Su S, et al. Minimally Invasive versus Open Thymectomy for Thymic Malignancies: Systematic Review and Meta-Analysis. J Thorac Oncol 2016;11:30-8. [Crossref] [PubMed]
- Iwanaka T, Arai M, Yamamoto H, et al. No incidence of port-site recurrence after endosurgical procedure for pediatric malignancies. Pediatr Surg Int 2003;19:200-3. [Crossref] [PubMed]
- Parekh K, Rusch V, Bains M, et al. VATS port site recurrence: a technique dependent problem. Ann Surg Oncol 2001;8:175-8. [Crossref] [PubMed]
- Downey RJ, McCormack P, LoCicero J. Dissemination of malignant tumors after video-assisted thoracic surgery: a report of twenty-one cases. The Video-Assisted Thoracic Surgery Study Group. J Thorac Cardiovasc Surg 1996;111:954-60. [Crossref] [PubMed]
- Walsh GL, Nesbitt JC. Tumor implants after thoracoscopic resection of a metastatic sarcoma. Ann Thorac Surg 1995;59:215-6. [Crossref] [PubMed]
- Wille GA, Gregory R, Guernsey JM. Tumor implantation at port site of video-assisted thoracoscopic resection of pulmonary metastasis. West J Med 1997;166:65-6. [PubMed]
- Sartorelli KH, Partrick D, Meagher DP. Port-site recurrence after thoracoscopic resection of pulmonary metastasis owing to osteogenic sarcoma. J Pediatr Surg 1996;31:1443-4. [Crossref] [PubMed]
- Kaiser GC. Practice guidelines in cardiothoracic surgery. The American Association for Thoracic Surgery, The Society of Thoracic Surgeons, the Southern Thoracic Surgical Association, the Western Thoracic Surgical Association. Ann Thorac Surg 1994;58:903-10. [Crossref] [PubMed]
- Anraku M, Nakahara R, Matsuguma H, et al. Port site recurrence after video-assisted thoracoscopic resection of chest wall schwannoma. Interact Cardiovasc Thorac Surg 2003;2:483-5. [Crossref] [PubMed]
- Jurado J, Javidfar J, Newmark A, et al. Minimally invasive thymectomy and open thymectomy: outcome analysis of 263 patients. Ann Thorac Surg 2012;94:974-81; discussion 981-2. [Crossref] [PubMed]
- Sakamaki Y, Oda T, Kanazawa G, et al. Intermediate-term oncologic outcomes after video-assisted thoracoscopic thymectomy for early-stage thymoma. J Thorac Cardiovasc Surg 2014;148:1230-7.e1. [Crossref] [PubMed]
- Khan IH, McManus KG, McCraith A, et al. Muscle sparing thoracotomy: a biomechanical analysis confirms preservation of muscle strength but no improvement in wound discomfort. Eur J Cardiothorac Surg 2000;18:656-61. [Crossref] [PubMed]
- Karnik PP, Dave NM, Garasia M. Comparison of analgesic efficacy and safety of continuous epidural infusion versus local infiltration and systemic opioids in video-assisted thoracoscopic surgery decortication in pediatric empyema patients. Saudi J Anaesth 2018;12:240-4. [Crossref] [PubMed]
- Piccioni F, Segat M, Falini S, et al. Enhanced recovery pathways in thoracic surgery from Italian VATS Group: perioperative analgesia protocols. J Thorac Dis 2018;10:S555-63. [Crossref] [PubMed]
- Butkovic D, Kralik S, Matolic M, et al. Postoperative analgesia with intravenous fentanyl PCA vs epidural block after thoracoscopic pectusexcavatum repair in children. Br J Anaesth 2007;98:677-81. [Crossref] [PubMed]
- Cook CH, Melvin WS, Groner JI, et al. A cost-effective thoracoscopic treatment strategy for pediatric spontaneous pneumothorax. Surg Endosc 1999;13:1208-10. [Crossref] [PubMed]
- Rothenberg SS, Chang JH, Toews WH, et al. Thoracoscopic closure of patent ductus arteriosus: a less traumatic and more cost-effective technique. J Pediatr Surg 1995;30:1057-60. [Crossref] [PubMed]
- Hess NR, Sarkaria IS, Pennathur A, et al. Minimally invasive versus open thymectomy: a systematic review of surgical techniques, patient demographics, and perioperative outcomes. Ann Cardiothorac Surg 2016;5:1-9. [PubMed]
- Smith TJ, Rothenberg SS, Brooks M, et al. Thoracoscopic surgery in childhood cancer. J Pediatr Hematol Oncol 2002;24:429-35. [Crossref] [PubMed]
- Hazelrigg SR, Boley TM, Krasna MJ, et al. Thoracoscopic resection of posterior neurogenic tumors. Am Surg 1999;65:1129-33. [PubMed]
- Bhatnagar S, Sarin YK. Scope and limitations of minimal invasive surgery in practice of pediatric surgical oncology. Indian J Med Paediatr Oncol 2010;31:137-42. [Crossref] [PubMed]
- Kern JA, Daniel TM, Tribble CG, et al. Thoracoscopic diagnosis and treatment of mediastinal masses. Ann Thorac Surg 1993;56:92-6. [Crossref] [PubMed]
- Kugler C. Minimal-invasive thoracic surgery in pediatric patients. J Vis Surg 2018;4:10. [Crossref] [PubMed]
- Partrick DA, Rothenberg SS. Thoracoscopic resection of mediastinal masses in infants and children: An evaluation of technique and results. J Pediatr Surg 2001;36:1165-7. [Crossref] [PubMed]
- Molinaro F, Garzi A, Cerchia E, et al. Thoracoscopic thymectomy in children: our preliminary experience. J Laparoendosc Adv Surg Tech A 2013;23:556-9. [Crossref] [PubMed]
- Miyagi J, Nagayoshi S. A case of diaphragm injury after insertion of the first trocar in VATS surgery. J Jpn Assoc Chest Surg 2016;30:255-8. [Crossref]
- Little D, Holcomb G III. Thoracoscopic biopsy of a mediastinal mass. In: Atlas of Pediatric Laparoscopy and Thoracoscopy with CD. Elsevier Health Sciences; 2008:275-83.
- Toker A, Sonett J, Zielinski M, et al. Standard terms, definitions, and policies for minimally invasive resection of thymoma. J Thorac Oncol 2011;6:S1739-42. [Crossref] [PubMed]
- Rothenberg SS. First decade’s experience with thoracoscopic lobectomy in infants and children. J Pediatr Surg 2008;43:40-4; discussion 45. [Crossref] [PubMed]
- Shigemura N, Shiono H, Inoue M, et al. Inclusion of the transcervical approach in video-assisted thoracoscopic extended thymectomy (VATET) for myasthenia gravis: a prospective trial. Surg Endosc 2006;20:1614-8. [Crossref] [PubMed]
- Petersen RH, Hansen HJ. Learning thoracoscopic lobectomy. Eur J Cardiothorac Surg 2010;37:516-20. [Crossref] [PubMed]
- Ponsky TA, Rothenberg SS, Tsao K, et al. Thoracoscopy in children: is a chest tube necessary? J Laparoendosc Adv Surg Tech A 2009;19:S23-25. [Crossref] [PubMed]
- Imperatori A, Rotolo N, Gatti M, et al. Peri-operative complications of video-assisted thoracoscopic surgery (VATS). Int J Surg 2008;6:S78-81. [Crossref] [PubMed]
- Taguchi T, Nagata K, Kinoshita Y, et al. The utility of muscle sparing axillar skin crease incision for pediatric thoracic surgery. Pediatr Surg Int 2012;28:239-44. [Crossref] [PubMed]
- Souzaki R, Kawakubo N, Miyoshi K, et al. The Utility of Muscle-Sparing Axillar Skin Crease Incision with Thoracoscopic Surgery in Children. J Laparoendosc Adv Surg Tech A 2018;28:1378-82. [Crossref] [PubMed]
- Hartwich J, Tyagi S, Margaron F, et al. Robot-assisted thoracoscopic thymectomy for treating myasthenia gravis in children. J Laparoendosc Adv Surg Tech A 2012;22:925-9. [Crossref] [PubMed]
- Hsu CP, Chuang CY, Hsu NY, et al. Comparison between the right side and subxiphoid bilateral approaches in performing video-assisted thoracoscopic extended thymectomy for myasthenia gravis. Surg Endosc 2004;18:821-4. [Crossref] [PubMed]
- Hsu CP. Subxiphoid approach for thoracoscopic thymectomy. Surg Endosc 2002;16:1105. [Crossref] [PubMed]
Cite this article as: Saikia J, Deo SVS, Bhoriwal S, Bharati SJ, Kumar S. Video assisted thoracoscopic surgery in paediatric mediastinal tumors. Mediastinum 2020;4:2.