
Long-term outcomes of mediastinoscopic esophagectomy in early esophageal squamous cell carcinoma: 269 cases study
Introduction
The morbidity of esophageal cancer is eighth in the world among cancer spectrum, the old populations are in higher risk. Even now this disease can be treated by multifarious approaches in every stage, such as operative treatment, radiotherapy, chemotherapy and immunization therapy. The prognosis is still poor, the 5-year survival rate is 16.9% (total) and 40% (after complete tumor resection) (1). Commonly esophageal cancer has two histological types including squamous cell carcinoma and adenocarcinoma. Esophageal squamous cell carcinoma is the major pathological pattern in East Asia, and about 53% (307,359 new cases) of all new cases of the world occurs in China, according to the Global Cancer Observatory (gco.iarc.fr) (2).
National Comprehensive Cancer Network (NCCN) and National Institute for Health and Care Excellence (NICE) published the treatment guidelines for esophageal squamous cell carcinoma in 2018. Upfront esophagectomy was referred for early stage (T1/2, less than 2 cm and well differentiated and no lymphatic metastasis) by NCCN/NICE. Two-phase, three-phase and transhiatal esophagectomy (THE) with standard or en-bloc lymphadenectomy are all acceptable in NCCN guideline (3). For the past few years, minimally invasive esophagectomy (MIE) was widely available. MIE might shorten the inpatient days, decrease the perioperative mortality and improve patient satisfaction (4). In a large data from the United States, MIE got similar 3-year survival rate compared with open esophagectomy (OE) (5).
Years ago, we reported mediastinoscopic esophagectomy (ME) can both maintain the integrity of the pleural cavity and enable mediastinal lymphadenectomy with lower requirements on the patients’ cardiopulmonary functions (6). However, this surgical approach was disputed, performed in a limited number of centers, it needed larger populations and long-term follow-up outcomes.
In November 2014, we improved the surgical process for resolving anastomotic fistula and recurrent nerve injury. The aim of this study was to report the postoperative complications and the long-term survival after ME for esophageal squamous cell carcinoma.
Methods
Patients
Ethics Committee of the Third Affiliated Hospital of Soochow University approved this study. A total of 269 patients underwent ME from December 2005 to March 2018, which were pathologically diagnosed with esophageal cancer by endoscopy in our center. Patients who were in stages cT1–2N0M0, primarily diagnosed by chest and abdominal computed tomography (CT) and endoscopic ultrasonography, were participated.
The patients were divided in two groups with the time line November 1st 2014 that we improved the surgical process at the cervical approach. The clinicopathological features of the patients in both groups, such as ME before or after Nov. 2014 were shown in Table 1. Differences in the clinicopathological features, postoperative complication and mortality, and the long-term clinical outcome after surgery were compared between the two groups.
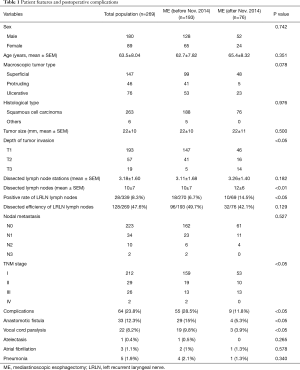
Full table
Surgical procedures
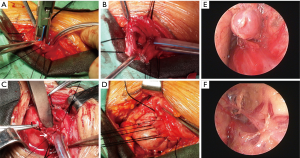
The operation requested a supine position, with the patients’ back padded and heads tilted. During general anesthesia a corrugated tube was used for endotracheal intubation. The neck process was operated along the left anterior sternocleidomastoid edge (up to the midpoint of the sternocleidomastoid and down to the jugular notch, about 6 cm long). Before Nov. 2014 we only isolated the part of the cervical esophagus under direct vision. After the time line we isolated both the followed cervical esophagus and the left recurrent laryngeal nerve (LRLN) under direct vision, and signed the LRLN with rubber rings for reducing the rates of vocal cord paralysis. The next step was the dissection of lymph nodes along the LRLN, the insertion of mediastinoscope and isolation of thoracic esophagus. The esophagus was then dissociated forwards, backwards, leftwards, and rightwards. The meche with pulling ropes was placed in four mediastinal positions as a marker, so as to ensure the esophagus was completely isolated. The trophic branch from the aorta for the esophagus was clipped using a titanium clip (or other hemostasis devices such as Harmonic and Ligasure) down to the level of the pulmonary veins. At the same time, the paraesophageal mediastinal lymph nodes were dissected. The tumid subcarinal lymph nodes were sampled with lymph node forceps for rapid intraoperative pathology. The stomach was dissociated via laparoscope or an anteromedian abdominal incision. Also, the lower esophagus was isolated through the diaphragmatic hiatus till the level of inferior pulmonary vein. After the complete isolation of the esophagus and stomach, we switched off the cardia with staples and then pulled out the esophagus from the cervical incision. After enlarging the esophageal hiatus, we sent the tubulous gastric body to the neck along the esophageal bed for cervical gastroesophageal anastomosis. A side-to-side stapled technique was improved by reinforcing the posterior wall of anastomosis with three stitches using 3-0 Vicryl Rapide suture. After the operation, every patient was sent to the intensive care unit of cardiothoracic surgery department for further monitoring and treatment (Figure 1).
Clinical outcome after treatment
In the first post-operative year, the patients were required to receive follow-up visit at the outpatient departments every three months, then every six months for follow-up visit. Chest and abdominal CT, neck/abdominal ultrasonography, and tumor markers were performed to check for any tumor recurrence/metastasis. If the patients did not visit the outpatient department timely, the doctor-in-charge would remind the patients for receiving follow-up visit. All data from follow-up results were entered into a medical record database. The follow-up data were retrieved to calculate the cumulative survival rate.
Statistical analysis
Differences between groups were studied by Chi-squared test, Mann-Whitney U test or t-test as appropriate. Survival was studied using the Kaplan-Maier method and the curve and trend was conducted by Log-rank (Mantel-Cox) test. Results were given as the mean ± SEM, median, percentage. A P value <0.05 was considered statistically significant. Analyses were conducted using GraphPad Prism 5.0 software or SPSS software 14.0 version (SPSS, Cary, NC, USA).
Results
The patients’ age, sex, macroscopic tumor type, histological type, tumor size, depth of tumor invasion, nodal metastasis, TNM stage were shown in Table 1. There were no differences between ME before or after Nov. 2014 groups. The dissected lymph node stations were 3.11±1.68 before Nov. 2014 and 3.26±1.40 after Nov. 2014. The averages of dissected lymph nodes were 10±7 before Nov. 2014 and 12±6 after Nov. 2014, while no differences were found between two groups. However, the positive rate of lymph nodes around LRLN after Nov. 2014 was more than the rate before Nov. 2014, 14.5% vs. 6.7% (P<0.05), and the ratio of LRLN lymph nodes in total dissected lymph nodes was 42.1% lower after Nov. 2014 compared to 49.7% before Nov. 2014 (P>0.05).
One-year survival
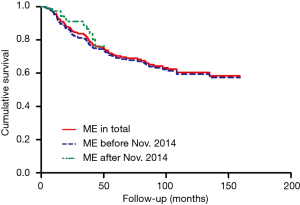
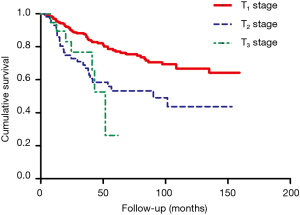
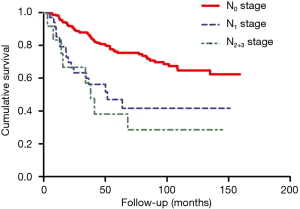
In this study, the overall 1-year survival was 95.5% in total patients, while the survival of ME after Nov. 2014 was 97.3% higher than 95.3% before Nov. 2014, Log-rank P value was 0.64 (Figure 2). The survival curve significantly differed among different T stages, T1 stage was better than T2 and T3 stage (Figure 3), more specifically, the 1-year survival was 95.9% at T1 stage, 93.0% at T2 stage, 94.7% at T3 stage. The survival curve significantly differed among different N stages, N0 stage was better than N1 and N2+3 stage (Figure 4), the 1-year survival was 97.3% at N0 stage, 85.3% at N1 stage, 83.3% at N2+3 stage.
Three-year survival
In this study, the overall 3-year survival was 82.0% in total patients, while the survival curve and trend were similar between ME before Nov. 2014 and after Nov. 2014, P=0.64 (Figure 2). The T1 stage survival curve significantly better than T2 and T3 stage (Figure 3), more specifically, the 3-year survival was 77.2% at T1 stage, 59.6% at T2 stage, 42.1% at T3 stage. The N0 stage survival curve significantly better than N1 and N2+3 stage (Figure 4), the 3-year survival was 75.3% at N0 stage, 50% at N1 stage, 50% at N2+3 stage.
Five-year survival
The overall 5-year survival was 69.2% in total patients, the survival curve and trend were similar between ME before Nov. 2014 and after Nov. 2014, P=0.64 (Figure 2). The survival curve significantly differed among different T stages (Figure 3), more specifically, the 5-year survival was 76.3% at T1 stage, 45.7% at T2 stage, 33.3% at T3 stage. In addition, the survival curve also significantly differed among different N stages (Figure 4), the 5-year survival was 79.1% at N0 stage, 53.8% at N1 stage, 66.7% at N2+3 stage.
Ten-year survival
The overall 10-year survival was 60.3% in total patients.
Postoperative complications
The total complications were 23.8% including 33 patients suffered anastomotic fistula, 22 patients suffered vocal cord paralysis, 1 patient suffered atelectasis, 3 patients suffered atelectasis, 5 patients suffer pneumonia. The complications in ME after Nov. 2014 were 11.8% significantly lower than 28.5% in ME before Nov. 2014 (P<0.05), due to lower morbidity of anastomotic fistula and vocal cord paralysis. Respectively, the anastomotic fistula was significantly decreased from 15% to 5.3% (P<0.05), and the vocal cord paralysis was significantly decreased from 9.8% to 3.9% (P<0.05) (Table 1). In this study, the incidence of the esophagogastrostomy leakage was 12.3% in total, no anastomotic fistula leaked inside upper posterior mediastinum. One patient died four months later because of cervical infection and hemorrhoea, others resolved with conservative treatment.
Discussion
Patients suffered esophageal squamous cell carcinoma usually had the history of cigarette smoking and alcohol consumption, then consequently combined with chronic diseases in those of the cardiovascular, pulmonary, and hepatic systems (7). The ME was resultful in highly risk esophageal cancer patients with serious comorbidities (6), and may decrease the morbidity and mortality (7).
In this series, the incidence of vocal cord paralysis was 8.2% after surgery in total population, although the previous reports described operative injuries of several organs during ME/THE, such as bronchial injury reported by Bumm et al. (8) and recurrent nerve injury in 36.6% of patients who underwent ME by Tangoku et al. (9). When the improved process for LRLN was applied, the rate decreased to 3.9% in 76 patients with ME after Nov. 2014, versus 9.8% in ME before Oct. 2014 (P<0.05). In the present study, two groups of medical staff were assigned to carry out cervical and abdominal operation simultaneously, so that the surgeons could carefully remove the esophagus surrounding by mediastinal lymph nodes for preserving the other organs, and the anesthesia time was decreased. In our experience, the use of suction cautery is associated with a high likelihood of recurrent laryngeal nerve injury. We considered it important to mark the LRLN with rubber rings before the cervical process, and careful work with powerless Ligasure system could prevent operative injury of the organs such as the recurrent nerve, bronchus, and trachea in these patients.
Anastomotic fistula inside thoracic cavity was one of the most serious post-operative complications, whose morbidity (10–20%) and mortality (4–50%) (10,11). If cervical leaks descended into the mediastinum without adequate drainage, significant mortality occurred, about 20% of patients would die (12). Even so, neck leakage still resulted in prolonged hospitalization, further interventions, and delayed oral nutrition and hydration. The posterior wall of anastomosis was usually weakest and the most common region of anastomotic leaks, therefore the surgeons enhanced this position with three stitches using 3-0 Vicryl Rapide suture. Depending on the improvement measure to the side-to-side stapled technique, the leakage rate significantly decreased to 5.3% after Nov. 2014 from 15% before Nov. 2014 in ME (P<0.05).
This study presented results showing a good survival after ME, 95.5% at 1 year, 82.0% at 3-year and 69.2% at 5-year, while the poor survival after OE was reported by other surgeons previously (13). The survival advantage might benefit from better staging and patient selection rather than the technique itself. Long-term follow-up showed that the overall survival was similar between thoracoscopic esophagectomy (TSE) and ME (14). In addition, lower morbidity and improved short-term survival after ME was presented for prolonging long-term survival (15). A population-based study from Finland and Sweden reported MIE improved 90-day survival (16), so was in current ME study for 99.6% at 90-day. The improved survival was possibly due to reduced morbidity and perfect living quality after ME.
The number of dissected lymph nodes was one of the independent predictors of survival, as N stage of esophageal cancer in the American Joint Commission on Cancer (AJCC) guidelines was judged primarily by the positive lymph nodes’ numbers (17). A minimum of removing 23 lymph nodes was recommended to improve the post-operative survival (18). In contrast, recent population-based studies shown that nothing was associated between the extent of lymphadenectomy and survival (19,20). In our series, no lymph node metastasis was confirmed by preoperative preparation. However, there were 28 lymph node metastases in 269 patients, yielding an average positive rate of 8.3%. Techniques for protecting LRLN also increased the positive rate of lymph nodes around LRLN, possibly due to higher dissecting accuracy or higher T-stages in the second group resulting in more positive lymph nodes. Furthermore, the long-term outcomes in this study indicated that N2–3 stage results in lower survival time, while the median survival of N2–3 stage was 36 months. Theoretically, the amplification effect of mediastinoscopy could help to achieve the complete resection of the enlarged mediastinal lymph nodes under direct vision (6). Due to the limited surgical field and angle, it was hardest to remove all mediastinal lymph nodes. Endoscopic techniques using carbon dioxide insufflation may resolve this question, it expanded the intra-mediastinal space in the upper mediastinum, especially near LRLN, while visualized the structures in the deep mediastinum around the aortic arch, such as nerves, bronchial arteries, and lymphatic vessels (21), allowed lymphadenectomy to be safely and carefully performed along the nerve (22).
In conclusion, the overall survival rate after ME was 60.3% at 10-year and 69.2% at 5-year, these findings supported the use of ME as an effective surgical method for esophageal cancer. The processes of isolating and marking the LRLN before the cervical process and reinforcing the posterior wall of anastomosis, were valuable for improvement of postoperative complications. More strict and accuracy perioperative preparations for N stage diagnose were required for scientific participation of patients. Additional studies in the technology of mediastinal carbon dioxide insufflation were needed.
Acknowledgments
We appreciate all patients who participated in this study.
Funding: This work was supported in part by Changzhou High-Level Medical Talents Training Project (grant numbers 2016CZBJ043) and the Applied Basic Research Programs of Changzhou (grant numbers CJ20159030, CJ20179040).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors Marcin Zielinski and Qingdong Cao for the series “Mediastinoscopic Surgery” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2019.08.01). The series “Mediastinoscopic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics Committee of the Third Affiliated Hospital of Soochow University approved this study. The number/ID of the approval was WZ200505. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol 2013;19:5598-606. [Crossref] [PubMed]
- Wang QL, Xie SH, Wahlin K, et al. Global time trends in the incidence of esophageal squamous cell carcinoma. Clin Epidemiol 2018;10:717-28. [Crossref] [PubMed]
- Wong I, Law S. The management of mid & proximal oesophageal squamous cell carcinoma. Best Pract Res Clin Gastroenterol 2018;36-37:85-90. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Yerokun BA, Sun Z, Yang CJ, et al. Minimally Invasive Versus Open Esophagectomy for Esophageal Cancer: A Population-Based Analysis. Ann Thorac Surg 2016;102:416-23. [Crossref] [PubMed]
- Wang QY, Li JP, Zhang L, et al. Mediastinoscopic esophagectomy for patients with early esophageal cancer. J Thorac Dis 2015;7:1235-40. [PubMed]
- Koide N, Takeuchi D, Suzuki A, et al. Mediastinoscopy-assisted esophagectomy for esophageal cancer in patients with serious comorbidities. Surg Today 2012;42:127-34. [Crossref] [PubMed]
- Bumm R, Feussner H, Bartels H, et al. Radical transhiatal esophagectomy with two-field lymphadenectomy and endodissection for distal esophageal adenocarcinoma. World J Surg 1997;21:822-31. [PubMed]
- Tangoku A, Yoshino S, Abe T, et al. Mediastinoscope-assisted transhiatal esophagectomy for esophageal cancer. Surg Endosc 2004;18:383-9. [Crossref] [PubMed]
- Parekh K, Iannettoni MD. Complications of esophageal resection and reconstruction. Semin Thorac Cardiovasc Surg 2007;19:79-88. [Crossref] [PubMed]
- Eroglu A, Turkyilmaz A, Aydin Y, et al. Current management of esophageal perforation: 20 years experience. Dis Esophagus 2009;22:374-80. [Crossref] [PubMed]
- Urschel JD. Esophagogastrostomy anastomotic leaks complicating esophagectomy: A review. Am J Surg 1995;169:634-40. [Crossref] [PubMed]
- Sihvo E, Helminen O, Gunn J, et al. Long-term outcomes following minimally invasive and open esophagectomy in Finland: A population-based study. Eur J Surg Oncol 2019;45:1099-104. [Crossref] [PubMed]
- Wang QY, Tan LJ, Feng MX, et al. Video-assisted mediastinoscopic resection compared with video-assisted thoracoscopic surgery in patients with esophageal cancer. J Thorac Dis 2014;6:663-7. [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Kauppila JH, Helminen O, Kyto V, et al. Short-Term Outcomes Following Minimally Invasive and Open Esophagectomy: A Population-Based Study from Finland and Sweden. Ann Surg Oncol 2018;25:326-32. [Crossref] [PubMed]
- Tsai TC, Miller J, Andolfi C, et al. Surgical evaluation of lymph nodes in esophageal adenocarcinoma: Standardized approach or personalized medicine? Eur J Surg Oncol 2018;44:1177-80. [Crossref] [PubMed]
- Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg 2008;248:549-56. [PubMed]
- Lagergren J, Mattsson F, Zylstra J, et al. Extent of Lymphadenectomy and Prognosis After Esophageal Cancer Surgery. JAMA Surg 2016;151:32-9. [Crossref] [PubMed]
- van der Schaaf M, Johar A, Wijnhoven B, et al. Extent of lymph node removal during esophageal cancer surgery and survival. J Natl Cancer Inst 2015;107. [PubMed]
- Ikeda Y, Niimi M, Kan S, et al. Thoracoscopic esophagectomy combined with mediastinoscopy via the neck. Ann Thorac Surg 2002;73:1329-31. [Crossref] [PubMed]
- Fujiwara H, Shiozaki A, Konishi H, et al. Single-Port Mediastinoscopic Lymphadenectomy Along the Left Recurrent Laryngeal Nerve. Ann Thorac Surg 2015;100:1115-7. [Crossref] [PubMed]
Cite this article as: Zheng L, Zhang X, Zhang L, Wang Q, Wang Z. Long-term outcomes of mediastinoscopic esophagectomy in early esophageal squamous cell carcinoma: 269 cases study. Mediastinum 2019;3:34.


