
Determining extent of invasion and follow-up of thymic epithelial malignancies
Introduction
Thymic epithelial malignancies (TEM), including thymoma, thymic neuroendocrine tumors and thymic carcinomas, are rare intrathoracic malignant tumors although they are the most common primary neoplasms of the anterior mediastinum (1). The goal of imaging TEM is to diagnose, stage, and follow up for recurrence and treatment response. While assessing for tumor staging, imaging studies are helpful in searching for locally advanced or disseminated disease, as patients with advanced disease will receive neoadjuvant therapy prior to surgery (2). Despite technologic advancements in different imaging modalities over the last few decades including chest radiography, computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET), in particularly with [18F] fluorodeoxyglucose (FDG), detection of tumor invasion is still challenging. Over this review, we will assess how these imaging modalities can be used for invasion assessment using direct and indirect signs. We will also review the proposed imaging follow-up after treatment.
Chest radiograph
Chest radiography is the initial imaging modality used when there is clinical suspicion of a mediastinal mass or TEM, with a detection rate of up to 80% of newly diagnosed TEM patients (3). TEM are depicted as a unilateral mediastinal mass with smooth or lobulated contours located anywhere from the thoracic inlet to the cardiophrenic angle. Although some forms of invasion or spread can be depicted with a chest radiograph, such as when there are large pleural metastases or when there is elevation of a hemidiaphragm as a result of phrenic nerve involvement, most metastases and/or local sites of invasion are too subtle to appreciate by chest radiography alone.
CT
CT is currently the imaging modality of choice for assessing TEM. CT is helpful in distinguishing TEM from other mediastinal tumors. It is useful for characterizing the primary tumor and staging the disease. CT demonstrates tumor location and morphology and is very helpful in detecting small tumors, which may present with a normal chest radiograph. CT readily displays the more aggressive direct features of TEM. For example, invasion of adjacent structures such as tumor invasion into adjacent vessels, the lung, chest wall, as well as pleural metastases or other distant organ metastases such as the lung, liver and bone. Just as in surgery, also with CT, it is often difficult to distinguish direct invasion from tumor adherence with no invasion, in particularly when assessing for local invasion into the mediastinal pleura or into the pericardium. Because of this, there have been attempts to find indirect signs for predicting local invasion and more distant metastatic disease. Initial studies focused on size based on surgery and assessed whether tumor size could predict loco regional spread or metastatic disease, demonstrated equivocal results (4-8). These studies were single institution small studies ranging from 58 (5) to 179 patients (8). Imaging studies have looked at size as well. Similar to the earlier surgical series, results of the CT series were equivocal (9-16), some showing a correlation of size with more advanced disease (12-16) others not (9-11). It is important to critically review these studies. They are all smaller studies from single institutions ranging from 45 (9) to 133 patients (11). Some studies correlated size with Masaoka stage I+II as compared to Masaoka stage III+IV (11-13,15), whereas others correlated Masaoka stage I to higher stages (II+III+IV) (14,16). Some incorporated into their statistics thymic carcinoma (9,10) and some did not (12,14,16). Only two of these studies (11,12) performed a multivariable analysis, and these two contradicted each other, one (12) showing a correlation of size with more advanced disease, the other (11), showing the size did not correlate with complete tumor resection. It was not until a more robust study was performed, using the International Thymic Malignancy Interest Group (ITMIG) retrospective database which had approximately 10,000 patients, that the tumor size issue was resolved. In this large cohort of patients, size did not correlate with more advanced disease or overall survival (17).
Aside from size, with CT, other imaging features of the primary tumor are readily appreciated and were assessed in small studies (12,14,16). Once again, the majority of these study lack a multivariable analysis. Of the two studies with multivariable analysis, a study looking at 99 thymoma patients (12) found that univariable analysis showed a correlation of advanced disease (Masaoka stage III+IV) with multiple imaging features of the primary tumor: when the primary tumor contained calcifications, was heterogeneous, lobulated, had infiltration of the fat surrounding it, had adjacent lung changes, abutted ≥50% of a vessel circumference. However, most of these did not survive the multivariable analysis. Only when the primary tumor was lobulated and infiltrated its surrounding fat, was it shown to be an independent predictor of advanced disease on the multivariable analysis. The other study with multivariable analysis (11) assessed 133 thymoma patients. It too, found multiple features of the primary tumor that correlated with advanced disease (Masaoka III+IV) in the univariable analysis, such as when the tumor was lobular, when fat planes around the primary tumor were lost, infiltration of fat surrounding the primary tumor, when the primary tumor abutted ≥50% of a vessel circumference, and when the tumor had adjacent lung changes. In this study, the authors only performed a multivariable analysis on the likelihood of these features to correlate with complete resection, and not surprisingly, many of these primary tumor features did not remain significant. In their multivariable analysis only when the primary abutted ≥50% of a vessel circumference, was it an independent predictor of an incomplete resection. Although these two studies are similar looking at their univariable analysis, they differ in trying to find an independent primary tumor prognostic marker for a worse outcome. They both suffer from the same issue, and that is, the small number of patients investigated. Unfortunately, there is no current data from a large TEM database that incorporated within it, detailed imaging information of the primary tumor. We hope that in the future, perhaps via the ITMIG prospective database, such detailed imaging features of the primary tumor will be incorporated, to help us better predict the clinical T stage, and help us better tailor the preoperative approach to the individual patient.
As far as nodal staging and distant metastatic staging, CT has a high sensitivity for detecting lesions suspicious for metastatic disease. However, as with any other staging, if such a lesion upstages the patient, a biopsy is recommended, as pulmonary nodules or liver nodules are not specific for metastatic disease and are often benign. In fact, the mere presence of pulmonary nodules in a staging CT for thymoma, does not correspond with advanced disease (11,12).
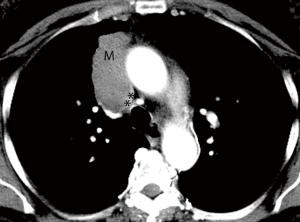
When ordering a chest CT for staging TEM, it is important to order and perform a contrast enhanced CT. The administration of contrast delineates the tumor borders better, enables evaluation of the vascular lumen and wall, all crucial for assessing local invasion (Figure 1). Intravenous contrast also helps in evaluating the upper abdomen for distant metastatic disease (Table 1).
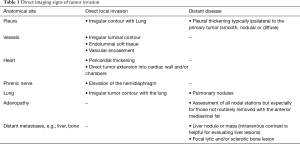
Full table
MRI
In general, CT is considered the imaging modality of choice for investigation of TEM detection and staging. In the past, MRI was reserved for those patients with a contraindication to iodinated contrast-material used with CT such as patients with an iodine allergy and/or renal failure (18). However, MRI is very effective in the evaluation of TEM. Although it’s spatial resolution is less than that of CT, its contrast resolution is superior to CT. This is particularly important in a disease like TEM, which tends to locally invade into mediastinal structures and through the pericardium and pleura. This improved contrast resolution is superior to CT in the detection of liver, and bone marrow metastases as well (Figure 2). There are few published studies on MRI’s capabilities of staging TEM as compared to CT. An early pilot study of 24 patients recently presented shows they are quite similar (19). A study that compared the staging capabilities of MRI when assessing another thoracic malignancy which spreads along the pleura and tends to locally invade the chest wall showed that CT and MRI were similar in their staging capabilities, but diaphragmatic invasion and focal chest wall invasion, were more readily detected by MRI (20). A study comparing the staging capabilities of MRI as compared to CT in 64 patients with TEM showed MRI to be superior to CT (21). Although one study assessing diffusion weighted imaging in 30 patients with TEM showed that statistically, lower apparent diffusion coefficient (ADC) values were associated with more advanced disease, the study incorporated thymic carcinoma patients which may have skewed the results. Even taking that into account, there was great overlap in these ADC values between early and more advanced disease making this clinically difficult to use (22). An additional study assessing 37 patients with TEM demonstrated that ADC analysis may assist in differentiating early versus advanced stage disease and different histologic subtypes (23). In another study of 41 TEM patients, ADC values could not discriminate early from more advanced staging (24).
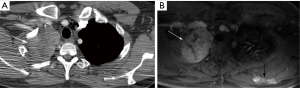
All of these published MRI studies are small, but initial data does suggest that MRI is at least as accurate as CT in staging TEM, and may have an advantage in select cases. Perhaps the greatest disadvantage of MRI is the length of the examination. Chest CT is acquired within a few seconds, and acquisition of a chest MRI currently takes 30 minutes or more. However, it is devoid of any ionizing radiation, its greatest advantage over CT. Thus, in select patient populations, in particularly in younger patients at risk for thymoma, such as patients with myasthenia gravis or multiple endocrine neoplasia type 1 annually screened for a thymic carcinoid, more thought should be given as to replacing the routine CT with MRI.
PET-CT
Integrated FDG PET/CT is an important diagnostic tool for the diagnosis, clinical staging, and outcome of intrathoracic malignancies, especially in patients with non-small cell lung cancer (25). However, the precise role of FDG PET/CT in the management of TEM, in particularly thymoma, is unclear. This is because some of the thymomas do not show increased FDG uptake. In such cases, when the primary tumor does not accumulate FDG, the study cannot improve staging. In specific cases, FDG PET-CT may be helpful in the histological differentiation of thymic epithelial neoplasms such as type B3 thymoma and thymic carcinoma, which tend to have high FDG uptake (26). Even when dealing with an FDG avid TEM, the poor spatial resolution of the PET component of the study, far worse than the spatial resolution of CT or MRI, cannot improve on local T staging. The strength of staging with FDG has to do with nodal and distant metastatic spread, as has been proven with other thoracic malignancies. There have been no large-scale studies comparing the ability of FDG PET/CT in staging TEM. A small study looking at 33 patients with TEM, only showed an advantage to staging with FDG in patients with thymic carcinomas (27). Unsuspected nodal involvement was detected by FDG uptake in 12% of patients with thymic carcinoma, missed by CT. One of the seven patients with pleural metastatic disease was detected by FDG PET, missed on the CT component of the study, again, a patient with thymic carcinoma (27).
In the past, a somatostatin analogue, Indium111 Octreotide was used for investigating TEM. However the amount of Indium111 Octreotide uptake is variable and does not correlate with tumor size, histological type, staging, clinical behavior or prognosis. Both planar imaging and SPECT imaging only detect deposits >1.5 cm and miss pleural and pericardial disease readily identified by CT and MRI. Imaging with Indium111 Octreotide has been replaced with other somatostatin analogues, which are used for PET imaging because of the improved spatial resolution they have leading to better detection (3–6 versus 10–15 mm) (28,29). An example of such 68Ga-labeled somatostatin analogues is: 68Ga-DOTA-TATE or 68Ga-DOTA-try-octreotide (DOTATOC). Such agents are not suitable for staging, and are reserved for second line therapy decisions when octreotide therapy is considered.
Follow-up imaging
Identification of TEM recurrence is important as early detection improves survival (30,31). The ITMIG official follow-up recommendations are based on the histologic classification of TEM and stage (32). The behavior of TEM changes drastically from some indolent forms of thymoma to the more aggressive thymic carcinoma. The average time to recurrence of a completely resected thymoma has been found to be approximately 5 years (range of reported average, 3–7 years) (4,33-39). It is because of this that the follow-up after TEM treatment is lengthy, spanning many years. When choosing an imaging modality for the follow-up of TEM, one should take into consideration the patient’s age and the effects of multiple ionizing radiation imaging studies and life expectancy according to histology and stage. ITMIG recommends at a minimum, annual chest CT scans for 5 years after surgical resection and then alternating annually with a chest radiograph until year 11, followed by annual chest radiographs alone (32). Resected stage III or IVa thymoma, thymic carcinoma, incomplete resection, or other high-risk tumors are suggested to undergo additional CT imaging every 6 months for 3 years. Obtaining a new “baseline” examination after resection when acute inflammatory effects have resolved (i.e., 4–12 weeks postoperatively) may be very useful for comparison. In this statement (32), ITMIG states that MRI may be useful instead of CT either for better visualization or to minimize cumulative radiation dose. It should be noted that there have been no published series assessing the accuracy of CT as compared to MRI in the follow-up of treated TEM patients. A recent retrospective study, presented (40) at the 9th ITMIG annual conference, assessed 22 patients with treated TEM who were followed with both CT and MRI. These imaging modalities showed relatively similar accuracies, with slight advantage of MRI in assessing pleural metastatic disease with direct involvement of the spinal canal, as well as an advantage in identifying bone marrow involvement. A disadvantage of MRI imaging after surgery was the use of sternal wires, which produced artifacts limiting the immediate retrosternal location at the level of these wires. Some of these artifacts were overcome by different MRI sequences, but because of this, the authors recommended alternating CT and MRI on follow-up. With the increasing popularity of minimally invasive surgical procedures, the number of patients undergoing tumor resection without sternal wires is expected to rise.
FDG PET-CT is not recommended for routine surveillance. This is predominantly due to the fact that thymomas may be non-FDG avid and the resolution of the PET component of the study is much lower than CT. However, in select cases, when a tumor is very aggressive with known high FDG uptake and a morphology difficult to technically measure, there may be some selective use for it, which may be considered on an individual basis, if it may change clinical management.
Conclusions
Thymic malignancies may exhibit aggressive behavior such as invasion of adjacent structures and involvement of the pleura and pericardium. The role of imaging in the evaluation of primary thymic neoplasms is to properly assess tumor staging, with emphasis on the detection of local invasion and distant spread of disease, correctly identifying candidates for preoperative neoadjuvant therapy. Different imaging modalities are used in the initial investigation of thymic malignancies including chest radiography, CT, MRI and FDG PET/CT. Although currently, CT has been the most commonly used imaging modality for staging and follow-up of TEM, there is an increasing trend to switch or alternate CT with MRI. Despite only small comparative studies available, it seems CT and MRI perform quite similarly in the staging and follow-up of TEM, with MRI being devoid of ionizing radiation.
Acknowledgments
The authors thank Kelly Kage for creating the table.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Editorial Office, Mediastinum for the series “Dedicated to the 9th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2018)” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: The series “Dedicated to the 9th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2018)” was commissioned by the editorial office without any funding or sponsorship. BWC serves as the unpaid Guest Editor of this series. BWC and MFB serve as an unpaid editorial board member of Mediastinum from May 2017 to Apr 2019 and from Jul 2019 to Jun 2021. BWC—Amirsys-Elsevier, Inc.: Thoracic content co-lead; EMM—lectured for: Bristol-Myers Squibb and Boehringer Ingelheim. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Morgenthaler TI, Brown LR, Colby TV, et al. Thymoma. Mayo Clin Proc 1993;68:1110-23. [Crossref] [PubMed]
- Falkson CB, Bezjak A, Darling G, et al. The management of thymoma: a systematic review and practice guideline. J Thorac Oncol 2009;4:911-9. [Crossref] [PubMed]
- Restrepo CS, Pandit M, Rojas IC, et al. Imaging findings of expansile lesions of the thymus. Curr Probl Diagn Radiol 2005;34:22-34. [Crossref] [PubMed]
- Blumberg D, Port JL, Weksler B, et al. Thymoma: a multivariate analysis of factors predicting survival. Ann Thorac Surg 1995;60:908-13; discussion 914. [Crossref] [PubMed]
- Elkiran ET, Abali H, Aksoy S, et al. Thymic epithelial neoplasia: a study of 58 cases. Med Oncol 2007;24:197-201. [Crossref] [PubMed]
- Gripp S, Hilgers K, Wurm R, et al. Thymoma: prognostic factors and treatment outcomes. Cancer 1998;83:1495-503. [Crossref] [PubMed]
- Nakagawa K, Asamura H, Matsuno Y, et al. Thymoma: a clinicopathologic study based on the new World Health Organization classification. J Thorac Cardiovasc Surg 2003;126:1134-40. [Crossref] [PubMed]
- Wright CD, Wain JC, Wong DR, et al. Predictors of recurrence in thymic tumors: importance of invasion, World Health Organization histology, and size. J Thorac Cardiovasc Surg 2005;130:1413-21. [Crossref] [PubMed]
- Kim JY, Kim HO, Kim JS, et al. (18)F-FDG PET/CT is Useful for Pretreatment Assessment of the Histopathologic Type of Thymic Epithelial Tumors. Nucl Med Mol Imaging 2010;44:177-84. [Crossref] [PubMed]
- Sadohara J, Fujimoto K, Muller NL, et al. Thymic epithelial tumors: comparison of CT and MR imaging findings of low-risk thymomas, high-risk thymomas, and thymic carcinomas. Eur J Radiol 2006;60:70-9. [Crossref] [PubMed]
- Hayes SA, Huang J, Plodkowski AJ, et al. Preoperative computed tomography findings predict surgical resectability of thymoma. J Thorac Oncol 2014;9:1023-30. [Crossref] [PubMed]
- Marom EM, Milito MA, Moran CA, et al. Computed tomography findings predicting invasiveness of thymoma. J Thorac Oncol 2011;6:1274-81. [Crossref] [PubMed]
- Ozawa Y, Hara M, Shimohira M, et al. Associations between computed tomography features of thymomas and their pathological classification. Acta Radiol 2016;57:1318-25. [Crossref] [PubMed]
- Priola AM, Priola SM, Di Franco M, et al. Computed tomography and thymoma: distinctive findings in invasive and noninvasive thymoma and predictive features of recurrence. Radiol Med 2010;115:1-21. [Crossref] [PubMed]
- Qu YJ, Liu GB, Shi HS, et al. Preoperative CT findings of thymoma are correlated with postoperative Masaoka clinical stage. Acad Radiol 2013;20:66-72. [Crossref] [PubMed]
- Tomiyama N, Muller NL, Ellis SJ, et al. Invasive and noninvasive thymoma: distinctive CT features. J Comput Assist Tomogr 2001;25:388-93. [Crossref] [PubMed]
- Nicholson AG, Detterbeck FC, Marino M, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the T Component for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S73-80. [Crossref] [PubMed]
- Carter BW, Benveniste MF, Truong MT, et al. State of the Art: MR Imaging of Thymoma. Magn Reson Imaging Clin N Am 2015;23:165-77. [Crossref] [PubMed]
- Benveniste M, Truong M, Betancourt Cuellar SL, et al. Oral 4.01: Comparison between computed tomography and magnetic resonance imaging in preoperative evaluation of thymic epithelial tumors. J Thorac Dis 2015;7:AB066.
- Patz EF Jr, Shaffer K, Piwnica-Worms DR, et al. Malignant pleural mesothelioma: value of CT and MR imaging in predicting resectability. AJR Am J Roentgenol 1992;159:961-6. [Crossref] [PubMed]
- Ohno Y, Kishida Y, Seki S, et al. Comparison of Interobserver Agreement and Diagnostic Accuracy for IASLC/ITMIG Thymic Epithelial Tumor Staging Among Co-registered FDG-PET/MRI, Whole-body MRI, Integrated FDG-PET/CT, and Conventional Imaging Examination with and without Contrast Media Administrations. Acad Radiol 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Abdel Razek AA, Khairy M, Nada N. Diffusion-weighted MR imaging in thymic epithelial tumors: correlation with World Health Organization classification and clinical staging. Radiology 2014;273:268-75. [Crossref] [PubMed]
- Kong LY, Zhang W, Zhou Y, et al. Histogram analysis of apparent diffusion coefficient maps for assessing thymic epithelial tumours: correlation with world health organization classification and clinical staging. Br J Radiol 2018;91:20170580 [Crossref] [PubMed]
- Priola AM, Priola SM, Giraudo MT, et al. Diffusion-weighted magnetic resonance imaging of thymoma: ability of the Apparent Diffusion Coefficient in predicting the World Health Organization (WHO) classification and the Masaoka-Koga staging system and its prognostic significance on disease-free survival. Eur Radiol 2016;26:2126-38. [Crossref] [PubMed]
- Antoch G, Stattaus J, Nemat AT, et al. Non-small cell lung cancer: dual-modality PET/CT in preoperative staging. Radiology 2003;229:526-33. [Crossref] [PubMed]
- Benveniste MF, Moran CA, Mawlawi O, et al. FDG PET-CT aids in the preoperative assessment of patients with newly diagnosed thymic epithelial malignancies. J Thorac Oncol 2013;8:502-10. [Crossref] [PubMed]
- Sung YM, Lee KS, Kim BT, et al. 18F-FDG PET/CT of thymic epithelial tumors: usefulness for distinguishing and staging tumor subgroups. J Nucl Med 2006;47:1628-34. [PubMed]
- Buchmann I, Henze M, Engelbrecht S, et al. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2007;34:1617-26. [Crossref] [PubMed]
- Lastoria S, Vergara E, Palmieri G, et al. In vivo detection of malignant thymic masses by indium-111-DTPA-D-Phe1-octreotide scintigraphy. J Nucl Med 1998;39:634-9. [PubMed]
- Regnard JF, Zinzindohoue F, Magdeleinat P, et al. Results of re-resection for recurrent thymomas. Ann Thorac Surg 1997;64:1593-8. [Crossref] [PubMed]
- Strobel P, Bauer A, Puppe B, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol 2004;22:1501-9. [Crossref] [PubMed]
- Huang J, Detterbeck FC, Wang Z, et al. Standard outcome measures for thymic malignancies. J Thorac Oncol 2010;5:2017-23. [Crossref] [PubMed]
- Huang J, Rizk NP, Travis WD, et al. Comparison of patterns of relapse in thymic carcinoma and thymoma. J Thorac Cardiovasc Surg 2009;138:26-31. [Crossref] [PubMed]
- Lewis JE, Wick MR, Scheithauer BW, et al. Thymoma. A clinicopathologic review. Cancer 1987;60:2727-43. [Crossref] [PubMed]
- Maggi G, Casadio C, Cavallo A, et al. Thymoma: results of 241 operated cases. Ann Thorac Surg 1991;51:152-6. [Crossref] [PubMed]
- Regnard JF, Magdeleinat P, Dromer C, et al. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg 1996;112:376-84. [Crossref] [PubMed]
- Ruffini E, Mancuso M, Oliaro A, et al. Recurrence of thymoma: analysis of clinicopathologic features, treatment, and outcome. J Thorac Cardiovasc Surg 1997;113:55-63. [Crossref] [PubMed]
- Verley JM, Hollmann KH. Thymoma. A comparative study of clinical stages, histologic features, and survival in 200 cases. Cancer 1985;55:1074-86. [Crossref] [PubMed]
- Wilkins KB, Sheikh E, Green R, et al. Clinical and pathologic predictors of survival in patients with thymoma. Ann Surg 1999;230:562-72; discussion 572-4. [Crossref] [PubMed]
- Kerpel A, Marom EM. AB013. OA02.04: MRI for the follow-up of treated thymic epithelial malignancies. Mediastinum 2018;2:AB013. [Crossref]
Cite this article as: Benveniste MF, Betancourt Cuellar SL, Carter BW, Shroff GS, Wu C, Marom EM. Determining extent of invasion and follow-up of thymic epithelial malignancies. Mediastinum 2019;3:29.


