
Thymic lesions of the paediatric age group: a comprehensive review of non-neoplastic and neoplastic etiologies
Introduction
The thymus, which is an anterior mediastinal organ, weighs approximately 25 g during birth. It reaches a maximum weight of 30–40 g during puberty and then undergoes involution but does not disappear completely. However, its percentage ratio to body weight is maximum at birth (0.76) and reaches upto 0.19 at around 5 years of age (1). Even the shape of the thymus changes with age where it is known to be quadrilateral shaped with convex margins at around 5 years of age and changes to triangular shape with straight margins by the age of 15 years (2).
Thymic lesions form approximately 2% of all mediastinal tumors in children (3). The rarity of thymic neoplasms in the paediatric age group has been highlighted in various studies conducted on a substantial number of cases of thymomas. In the report by European Cooperative Study Group for Pediatric Rare Tumors (EXPeRT) only 36 cases of paediatric thymic neoplasms could be registered over a 12-year period across four European nations (4). Even in one of the largest series of 1,470 patients of thymomas carried out by the Thymic International Group spanning across 14 institutions of 11 countries, none of the cases were below 12 years of age (5). A recent study from our institute on 84 cases of thymomas also did not have patients of paediatric age group in the study cohort (6). The clinical profile of these patients varies from asymptomatic incidentally detected masses to being symptomatic with dyspnoea, dysphagia or venous congestion (7). The myriad paraneoplastic syndromes (PNS) associated with the thymus such as myasthenia gravis (MG), pure red cell aplasia, hypogammaglobulinemia, pemphigus, Sjogren’s syndrome manifest more commonly in adults rather than in childhood (1,4,8). Besides the ones mentioned above, other clinical manifestations include gastrointestinal disorders (chronic ulcerative colitis), collagen and autoimmune disorders (scleroderma and polymyositis), dermatologic disorders (alopecia), endocrine disorders (Cushing’s syndrome), renal disease (nephrosis) and hematologic syndromes (agranulocytosis) (4). It has also been seen that MG is the most common PNS and is generally seen commonly in children <10 years of age (7). In a study on juvenile MG, it was found that therapeutic thymectomy revealed 97.5% of the patients to be harbouring thymic hyperplasia and even thymomas despite the thymus being radiologically normal in size (9). Some rare associations of thymoma have been reported in literature and include the occurrence of nevus sebaceous (10).
The Tumori Rari in Età Pediatrica (Rare Tumours in Paediatric Age) (TREP) project launched in Italy in the year 2000 to encourage research on rare paediatric malignancies have formulated guidelines to diagnose paediatric thymomas (11). For initial assessment, a chest X-ray followed by a computed tomography (CT) scan and/or a magnetic resonance imaging (MRI) was suggested to assess the extent of local involvement by the lesion. Further, a bone marrow biopsy was suggested to rule out lymphoid neoplasms.
Radiological classification
Radiologists have attempted to classify thymic lesions in children according to the density of the visible mass into solid, fatty and cystic lesions (2). A common differential of a solid thymic mass in a child is a prominent normal thymus which is also referred to as a “pseudomass” since it actually isn’t a tumor but the normal appearance of thymus in that particular age group (2). The other solid lesions are ectopic thymus which may be retrocaval (between the superior vena cava and the great arteries) or cervical, thymic hyperplasia or thymic neoplasms. The cystic lesions include thymic cysts and fatty lesions are the thymolipomas.
The other method by which radiologists have classified paediatric thymic lesions are based on the size of thymus whether it is small or large (12). Small thymus can be physiological which may be age related involutions or treatment related atrophy or immunodeficiency disorders leading to hypoplasia or aplasia. Large thymus included hyperplasias and neoplastic lesions.
Thymic hyperplasia
Thymic hyperplasias can be true thymic hyperplasia or lymphoid (follicular) hyperplasia. True thymic hyperplasias result in change in the shape of the gland as well as increase in the size which may be more than 50% of original (12). The hyperplasia is seen in patients recovering from various stressors such as burns, major surgeries, infections, post therapies which may be corticosteroids, radiation or chemotherapy or they may have other related disorders such as hyperthyroidism or sarcoidosis. Thymic hyperplasia may present as an emergency with symptoms of respiratory stridor, dysphagia, cyanosis and dyspnoea (13). During a stressful situation, the thymus shrinks in volume by about 40% and then again regrows to reach the original size or even larger. This phenomenon is known as rebound hyperplasia which is the most common form occurring in paediatric population (14-16). Rebound hyperplasia most commonly appear within two years of initiation of chemotherapy in many malignancies and are thought to be indicators of cell mediated immunity thereby, representing better prognosis in such children (15). Some studies have even defined the architectural features of thymus on CT images which point towards rebound hyperplasias namely “triangular shaped, bilobulated, homogeneous soft tissue with convex contour in the normal thymic region” (15) or a 50% increase in thymic volume over baseline (16). Thymic hyperplasias are also found commonly in male patients and are associated with peripheral lymphocytosis (17). Clinically patients of true thymic hyperplasia may have a related pre-existing condition, may be recovering from recent stressors such as fever, pneumonia or therapy (14,18). Follicular hyperplasia shows predominantly increased lymphoid follicles without substantial increase in the size of the gland and has been known to be associated with immunologically mediated disorders such as MG, vasculitis, thyrotoxicosis and Grave’s disease (12) and also early stages of human immunodeficiency virus infection (14).
Status thymolymphaticus
The condition of “Status Thymolymphaticus” is of historical interest in the early 20th century, where necropsy on patients of sudden death without preceding stridor revealed enlarged thymus which was thought to be due to lymphatic diathesis (13). The enlarged thymus was believed to cause respiratory difficulties in situations of minor accidents, light anaesthesia or small surgeries leading to sudden death (19). This concept was refuted by Levine and Rosai in their article where they called it an erroneous interpretation of an essentially normal sized thymus causing respiratory obstruction (20). They had advised comparison of the volume of thymus against a standard measurement prepared from thymic tissues obtained from autopsies of individuals with sudden death to label them as hyperplasias.
Thymic cysts
Thymic cysts (Figure 1A,B) are uncommon among the paediatric population forming less than 1% of all mediastinal masses and may be congenital or acquired. The congenital cysts also arise along the thymopharyngeal duct when the thymus undergoes atrophy and the remnants undergo cystic change (14). They present suddenly as painless neck masses and are commoner on the left side of neck (21), more prevalent on the anterior surface of sternocleidomastoid and around 50% of them may extend to the superior mediastinum (22). The acquired cysts may arise post radiotherapy in a case of Hodgkin or non-Hodgkin lymphoma or as inflammatory cysts seen in autoimmune disorders (12,14). They may also arise post thoracotomy or may be associated with thymic tumours that cause distortion and compression of surrounding normal thymic tissue (23). The congenital cysts are usually unilocular while those arising in the thymus are multilocular arising due to degeneration of Hassal’s corpuscles (22). The multilocular thymic cysts were common in the era when anti-retroviral therapy was not available (14). The unilocular thymic cysts are thin walled containing serous fluid and the multilocular ones have thick wall with fibrous adhesions and contain turbid or hemorrhagic material. A proliferating multilocular thymic cyst has also been described which histologically may mimic an epidermoid or a trichilemmal cyst and may be mistaken for a cystic squamous cell carcinoma (24). The differential diagnoses of cervical thymic cyst include thyroglossal duct cyst, branchial cleft cyst, laryngocele, lymphovascular malformations, benign tumors (dermoid and epidermoid cysts) and malignant tumors. Thymic cysts are treated surgically and complete excision do not cause recurrences in children (22). An important differential of thymic cysts is a cystic thymoma which is difficult to distinguish radiologically. The histopathological features clinch the diagnosis and include absence of epithelial lining of the cyst wall, solid areas in the wall comprising of epithelial cells and lymphoid cells and perivascular spaces along with medullary differentiation at some foci (25).
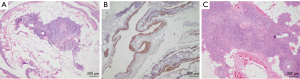
Thymolipomas
Thymolipomas (Figure 1C) are benign neoplasms comprising of normal thymic tissue interspersed with fat and fibrous septae. Due to their fat content, they are extremely pliable and may sometimes occupy the entire thoracic cavity (23). They may be incidentally detected, or the patients may present with compression symptoms or associated PNS. They are encapsulated non infiltrating lesions located mostly in the inferior part of the anterior mediastinum. Its diagnosis can be proposed radiologically itself due to its appearance of a fatty mass, location and continuity with the thymus. However, the differential diagnoses include other fatty masses such as liposarcoma or a teratoma (26). At times the lipomatous component of the thymolipoma may undergo sarcomatous change and may resemble a well-differentiated or pleomorphic liposarcoma (24). Thymomas may also occasionally arise within a thymolipoma (27).
Thymic epithelial neoplasms
Thymoma
Thymomas are extremely rare in children forming less than 5% of all mediastinal masses. They are usually asymptomatic and are detected incidentally on routine chest radiography. They are classified according to the World Health Organization (WHO) classification of thymic tumors and are staged in accordance with the modified Masaoka Koga staging system. The rarity of the disease has caused difficulty in ascertaining prognosis or formulating treatment strategies of this entity in children and adolescents (7). Earlier studies on thymomas in children encompassing limited number of cases along with review of available literature have revealed younger age group (<10 years) patients to have a male predominance, being in advanced stage and having less favourable outcome as compared to adults (17). In contrast, patients of age more than 10 years have shown a reciprocal association with predominance of female gender, lower stage of disease and comparatively better outcome (17). The reason speculated for a worse outcome at a younger age was the improperly developed immune system leading to tumor dissemination. However, case reports of good outcome in thymoma in patients <10 years of age do exist in literature (7). In addition to stage and histologic subtype, the type of paraneoplastic condition associated with thymic neoplasms also have prognostic significance (17). The incidence of MG associated with thymomas is more common in adults as compared to children (10,28). The main differential diagnoses of paediatric thymomas are true thymic hyperplasia, precursor T-lymphoblastic lymphoma (TLBL), and thymic carcinoma.
Thymic carcinomas
Thymic carcinomas are very rare tumors in adults and are even rarer in the paediatric age group forming less than 1% of all childhood neoplasms (29,30). They are less likely to present with MG or other PNS as compared to thymomas (31) and have other constitutional symptoms as well including chest pain, weight loss, fatigue, night sweats, symptoms of superior vena caval compression and recurrent lower respiratory tract infections (2,32). They generally have a rapidly progressive clinical course (33). They form large irregular masses with local spread and lymphovascular invasion (2) and show rapid evolution as compared to adults (33). The thymic carcinomas arise from the thymic epithelial cells which lose their functional and phenotypic features and appear anaplastic (3). The recent 2015 WHO classification of tumors of lung, pleura, thymus and heart (34) have described many histopathological subtypes of thymic epithelial carcinomas comprising of squamous cell carcinoma, basaloid carcinoma, mucoepidermoid carcinoma, lymphoepithelioma-like carcinoma (LELC), clear cell carcinoma, sarcomatoid carcinoma, nuclear protein in testis (NUT) carcinoma and the undifferentiated carcinoma. Of these, the NUT carcinoma, LELC and the mucoepidermoid carcinomas have been described in the paediatric age group. But due to the extreme rarity of these malignancies, only case reports exist in literature (29). The carcinoma cells are immuno-positive for CD5 and CD117 and are mostly higher stage tumors. The most common sites of metastases are lung, liver, bone and kidneys with rare intracranial metastases in child (31).
The standard treatment protocols as recommended by the National Comprehensive Cancer Network (NCCN) (35) for resectable or unresectable thymomas/thymic carcinomas have not varied between adults or children. Total thymectomy and complete excision of the tumor is recommended for all operable patients and completeness of resection acts as the most important predictive parameter for outcome. Earlier studies have shown similar outcomes in adults and children in lower stage thymomas treated by complete excision alone (11,17). Post-operative radiotherapy is the recommended adjuvant treatment in incompletely resected cases. Its role in the paediatric age group is debatable due to subsequent toxicity on the lungs and heart, however, the EXPeRT has proposed consideration of this modality of treatment in this age group also (4). Chemotherapy is offered in cases of thymic carcinomas or thymomas with R2 resection status. The recommended first line chemotherapy preferred for thymomas are CAP (cyclophosphamide, adriamycin, cisplatin) regimen or a combination of etoposide, ifosfamide and cisplatin whereas carboplatin and paclitaxel are preferred for thymic carcinomas. Researchers have varied the chemotherapy protocols in children as per their institutional practices (17,28) and have shown better response including the EXPeRT group where they have found the regimen comprising cisplatin, etoposide and ifosfamide to be the most beneficial (4). The Polish Paediatric Rare Tumor Study have also used a multi-drug regimen derived from various treatment protocols and have used cisplatin and ifosfamide but have suggested further exploration of chemotherapy for treatment of thymic carcinomas in children (32). Targeted therapy using tyrosine kinase inhibitors such as imatinib and dasatinib have shown promising results especially in patients with strong CD117 expression (11,32).
Thymic carcinoid
The thymic carcinoids were first characterised by Rosai and Higa in 1972 where they called 11 such cases as “carcinoid tumors of the thymus (CTT)” (24). Their neuroendocrine nature was based on the electron microscopy findings as well as their association with multiple endocrine neoplasia 1 (MEN1) syndrome and ectopic Cushing’s syndrome. Thymic carcinoids are also rare with an incidence of 2.6–8% (36). They are exclusively found in males. These carcinoids are known to have increased morbidity with presence of local spread, distant metastasis and recurrence. Histopathologically, they form a part of the spectrum of neuroendocrine tumors of the thymus described in the 2015 WHO classification, which encompass the typical and atypical carcinoids and the high grade neuroendocrine tumors including the small cell and large cell neuroendocrine carcinomas. These tumors are also associated with PNS. Case reports in children have revealed association with ectopic production of ACTH leading to Cushing’s syndrome (37).
Thymic lymphomas
Lymphomas form the most common anterior mediastinal mass in children and thymic involvement by lymphomas can occur commonly in disseminated disease as compared to primary thymic origin. Among the histopathological subtypes, Hodgkin lymphoma forms the most common variant (23). The lymphoblastic lymphomas may be difficult to distinguish from type B thymomas and may even have leukemic potential (33).
Small thymus
Small thymus is seen in children either due to physiological cases or in cases of immunodeficiency disorders. The physiological causes include age related involution, treatment related atrophy as well as presence of ectopic thymus.
Thymic involution
Besides the physiological age-related involution, its existence in the paediatric age group can also be as an after effect of stress which exert immunosuppressive effects. The causes of stress related involution include malnutrition, illness, chemotherapy, corticosteroid therapy, panhypogammaglobulinemia and polymicrobial infections (38). Thymus is greatly affected by the nutritional status of the child (39) such that an involuted thymus of a malnourished child is said to have undergone a “nutritional thymectomy. Similarly, difference in thymic sizes have been noted in children who are breast fed as compared to those who are formula fed wherein hyperplasia is noted in the former in comparison to latter (38). Involution has also been identified in children who are neglected or abused and the degree of involution corresponded to the period of abuse/ neglect (40). As involution is rare in the early childhood, its occurrence at that age can be taken as an indicator of abuse/neglect. The process of involution commences with depletion of cortical lymphocytes leading to “lymphocyte inversion” where the medullary region starts appearing lymphocyte dense. The medulla is the last component to involute. The left-over epithelial cells tend to aggregate together and may appear “pseudo-atypical” and mimic a metastasis or a thymic carcinoma. As the age commences, the amount of admixed adipocytes increase in quantity (41).
Ectopic thymus
The ectopic thymus arises along the thymopharyngeal tract extending from the neck to the anterior mediastinum due to sequestration, incomplete descent or failure to involute (14,23). The other rare sites of ectopic thymus are the posterior mediastinum or the dermis.
Atrophy
Thymic atrophy is defined as a loss in cellularity of the thymus and occurs commonly as a physiological phenomenon of aging. Atrophy in children can occur due to stress, infections and chemo-radiotherapy. Infections causing depletion of thymocytes may be bacterial such as Mycobacterium avium or Salmonella typhimurium, viral such as severe influenza A (H1N1) or HIV infection of the thymocytes causing depletion of CD4 cells (42).
Immunodeficiency disorders such as DiGeorge syndrome are associated with thymic aplasia/hypoplasia and cause severe T-cell lymphopenia exposing the child to a plethora of infections (43). Multiple etiologies of DiGeorge syndrome are known of which 22q11.2 deletions form the most frequent cause (44). Thymic transplants have also been attempted on experimental basis in these cases (43).
Conclusions
The thymus forms an important part of the immune system of the paediatric age group. Thymic lesions are rare causes of anterior mediastinal pathology with occurrence being even rarer in the children. Nevertheless, knowledge about their pathologies in children become important as they differ from adults not only in the clinical presentation but also in the pathogenesis and natural disease history. This review aspires to bring about a comprehensive overview of thymic pathology in the paediatric age group.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Editorial Office, Mediastinum for the series “Pediatric Mediastinal Tumors” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2019.05.04). The series “Pediatric Mediastinal Tumors” was commissioned by the editorial office without any funding or sponsorship. DJ serves as an unpaid editorial board member of Mediastinum from Aug 2018 to Aug 2020 and serves as the unpaid Guest Editor of this series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ocal T, Türken A, Ciftçi AO, et al. Thymic enlargement in childhood. Turk J Pediatr 2000;42:298-303. [PubMed]
- Ranganath SH, Lee EY, Restrepo R, et al. Mediastinal Masses in Children. AJR Am J Roentgenol 2012;198:W197-216 [Crossref] [PubMed]
- Yaris N, Nas Y, Cobanoglu U, et al. Thymic carcinoma in children. Pediatr Blood Cancer 2006;47:224-7. [Crossref] [PubMed]
- Stachowicz-Stencel T, Orbach D, Brecht I, et al. Thymoma and thymic carcinoma in children and adolescents: A report from the European Cooperative Study Group for Pediatric Rare Tumors (EXPeRT). Eur J Cancer 2015;51:2444-52. [Crossref] [PubMed]
- Weissferdt A, Kalhor N, Bishop JA, et al. Thymoma: a clinicopathological correlation of 1470 cases. Hum Pathol 2018;73:7-15. [Crossref] [PubMed]
- Guleria P, Husain N, Shukla S, et al. PD-L1 immuno-expression assay in thymomas: Study of 84 cases and review of literature. Ann Diagn Pathol 2018;34:135-41. [Crossref] [PubMed]
- Saha S, Suhani S, Basak A, et al. Pediatric Thymoma with a Difference: Report of a Case and Review of Literature. J Surg Tech Case Rep 2014;6:64. [Crossref] [PubMed]
- Myers JL. Mediastinum. In: Goldblum J, Lamps L, McKenney J, et al. editors. Rosai and Ackerman’s Surgical Pathology. Philadelphia: Elsevier, 2018:460-81.
- Vázquez-Roque FJ, Hernández-Oliver MO, Medrano PY, et al. Resultados del tratamiento quirúrgico en la miastenia gravis juvenil. Neurología 2017;32:137-42. [Crossref] [PubMed]
- Dhall G, Ginsburg HB, Bodenstein L, et al. Thymoma in children: report of two cases and review of literature. J Pediatr Hematol Oncol 2004;26:681-5. [Crossref]
- Carretto E, Inserra A, Ferrari A, et al. Epithelial thymic tumours in paediatric age: a report from the TREP project. Orphanet J Rare Dis 2011;6:28. [Crossref] [PubMed]
- Manchanda S, Bhalla AS, Jana M, et al. Imaging of the pediatric thymus: Clinicoradiologic approach. World J Clin Pediatr 2017;6:10-23. [Crossref] [PubMed]
- Mitchell AG, Warkany J. The problem of the thymus in children. JAMA 1939;112:283-5. [Crossref]
- Nasseri F, Eftekhari F. Clinical and Radiologic Review of the Normal and Abnormal Thymus: Pearls and Pitfalls. RadioGraphics 2010;30:413-28. [Crossref] [PubMed]
- Arpaci T, Karagun BS. Rebound thymic hyperplasia after bone marrow transplantation in children with haemato-oncological diseases. Contemp Oncol (Pozn) 2018;22:95-8. [Crossref] [PubMed]
- Choyke PL, Zeman RK, Gootenberg JE, et al. Thymic atrophy and regrowth in response to chemotherapy: CT evaluation. AJR Am J Roentgenol 1987;149:269-72. [Crossref] [PubMed]
- Liang X, Lovell MA, Capocelli KE, et al. Thymoma in Children: Report of 2 Cases and Review of the Literature. Pediatr Dev Pathol 2010;13:202-8. [Crossref] [PubMed]
- Mishra SK, Melinkeri SR, Dabadghao S. Benign thymic hyperplasia after chemotherapy for acute myeloid leukemia. Eur J Haematol 2001;67:252-4. [Crossref] [PubMed]
- King JC. Thymic Enlargement in Children: Its Diagnosis and Treatment. Radiology 1927;9:148-52. [Crossref]
- Levine GD, Rosai J. Thymic hyperplasia and neoplasia: a review of current concepts. Hum Pathol 1978;9:495-515. [Crossref] [PubMed]
- Prosser JD, Myer CM. Branchial Cleft Anomalies and Thymic Cysts. Otolaryngol Clin North Am 2015;48:1-14. [Crossref] [PubMed]
- Sturm JJ, Dedhia K, Chi DH. Diagnosis and Management of Cervical Thymic Cysts in Children. Cureus 2017;9:e973 [PubMed]
- Goldstein AJ, Oliva I, Honarpisheh H, et al. A tour of the thymus: a review of thymic lesions with radiologic and pathologic correlation. Can Assoc Radiol J 2015;66:5-15. [Crossref] [PubMed]
- Wick MR. Mediastinal pathology and the contributions of Dr. Juan Rosai. Semin Diagn Pathol 2016;33:319-32. [Crossref] [PubMed]
- Honda S, Morikawa T, Sasaki F, et al. Cystic thymoma in a child: a rare case and review of the literature. Pediatr Surg Int 2007;23:1015-7. [Crossref] [PubMed]
- Calandriello L, Larici AR, Sica G, et al. The added value of chemical shift MRI in the preoperative diagnosis of thymolipoma. Tumori 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Yvorel V, Forest F, Parietti E, et al. B3 thymoma arising within thymolipoma. Pathology 2015;47:702-5. [Crossref] [PubMed]
- Fonseca AL, Ozgediz DE, Christison-Lagay ER, et al. Pediatric thymomas: report of two cases and comprehensive review of the literature. Pediatr Surg Int 2014;30:275-86. [Crossref] [PubMed]
- Sekihara K, Okuma Y, Kawamoto H, et al. Clinical outcome of thymic lymphoepithelioma-like carcinoma: Case report of a 14-year-old male. Oncol Lett 2014;8:2183-6. [Crossref] [PubMed]
- Ferrari A, Bisogno G, De Salvo GL, et al. The challenge of very rare tumours in childhood: The Italian TREP project. Eur J Cancer 2007;43:654-9. [Crossref] [PubMed]
- Kumar N, Chaudhary N, Prabhu AJ, et al. Undifferentiated thymic carcinoma with intracranial metastasis in a two-year-old. Asian Cardiovasc Thorac Ann 2018;26:239-41. [Crossref] [PubMed]
- Stachowicz-Stencel T, Bien E, Balcerska A, et al. Thymic carcinoma in children: A report from the Polish pediatric rare tumors study. Pediatr Blood Cancer 2010;54:916-20. [Crossref] [PubMed]
- Spigland N, Di Lorenzo M, Youssef S, et al. Malignant thymoma in children: a 20-year review. J Pediatr Surg 1990;25:1143-6. [Crossref] [PubMed]
- Chan JKC, Ströbel P, Marx A et al. Thymic carcinomas. In: Travis WD, Brambilla E, Burke AP, et al. editors. WHO classification of tumors of the lung, pleura, thymus and heart. Lyon, France: IARC, 2015:212-33.
- National Comprehensive Cancer Network. Thymomas and Thymic Carcinomas (Version 2.2019). Accessed April 22, 2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/thymic.pdf
- Singh Ospina N, Thompson GB. Thymic and Bronchial Carcinoid Tumors in Multiple Endocrine Neoplasia Type 1: The Mayo Clinic Experience from 1977 to 2013. Horm Cancer 2015;6:247-53. [Crossref] [PubMed]
- Soltysiak J, Ostalska-Nowicka D, Zaorska K, et al. Atypical thymic carcinoid manifesting with nephrotic-range proteinuria in a 7-year-old boy. Pediatr Nephrol 2017;32:1465-8. [Crossref] [PubMed]
- Prentice AM, Collinson AC. Does breastfeeding increase thymus size? Acta Paediatr 2000;89:8-12. [Crossref] [PubMed]
- Rytter MJH, Namusoke H, Ritz C, et al. Correlates of thymus size and changes during treatment of children with severe acute malnutrition: a cohort study. BMC Pediatr 2017;17:70. [Crossref] [PubMed]
- Fukunaga T, Mizoi Y, Yamashita A, et al. Thymus of abused/neglected children. Forensic Sci Int 1992;53:69-79. [Crossref] [PubMed]
- Raica M, Cîmpean AM, Encică S, et al. Involution of the thymus: a possible diagnostic pitfall. Rom J Morphol Embryol 2007;48:101-6. [PubMed]
- Majumdar S, Nandi D. Thymic Atrophy: Experimental Studies and Therapeutic Interventions. Scand J Immunol 2018;87:4-14. [Crossref] [PubMed]
- Amatuni GS, Currier RJ, Church JA, et al. Newborn Screening for Severe Combined Immunodeficiency and T-cell Lymphopenia in California, 2010-2017. Pediatrics 2019;143:e20182300 [Crossref] [PubMed]
- McDonald-McGinn DM, Sullivan KE, Marino B, et al. 22q11.2 deletion syndrome. Nat Rev Dis Prim 2015;1:15071. [Crossref] [PubMed]
Cite this article as: Guleria P, Jain D. Thymic lesions of the paediatric age group: a comprehensive review of non-neoplastic and neoplastic etiologies. Mediastinum 2019;3:24.


