
Approach to the prevascular mass
Introduction
On the basis of discussions with experts in the field of mediastinal diseases, the International Thymic Malignancy Interest Group (ITMIG) has introduced a new definition of mediastinal compartments to be used with cross-sectional imaging and adopted as a new standard (1). This clinical classification defines a 3-compartment model of prevascular (anterior), a visceral (middle), and a paravertebral (posterior) compartment, with anatomic boundaries defined clearly by computed tomography.
Thymic cyst
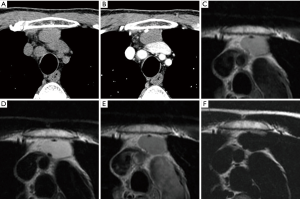
Simple congenital thymic cysts usually appear as well-defined water-attenuation masses with imperceptible walls. Multilocular thymic cysts may appear as well-defined, heterogeneous, multilocular cystic masses with a clearly seen wall (2). However, some thymic cysts may have an increased CT attenuation if hemorrhage or infection occurs as a complication and may be misdiagnosed as solid masses (Figure 1A,B). MRI is useful in this situation. By using water-sat sequence, thymic cysts can be diagnosed with confidence (Figure 1C,D,E,F). Of course, any thymic cysts show no enhancement on CT or MRI. Curvilinear calcification of the cyst wall occurs in a minority of cases.
Thymoma
Thymoma, even when invasive, typically manifests as a well-defined, spherical or lobulated soft-tissue mass located in the region of the thymus (3). Thymoma may show thin curvilinear capsular calcification and may be separated by thick fibrous septa (4-6). Tumor heterogeneity with areas of low attenuation representing hemorrhage, necrosis, or cystic formation may be a dominant feature. Mass usually enhances uniformly after intravenous contrast administration, unless necrosis and hemorrhage are present (7). Cross-sectional imaging findings highly suggestive of capsular invasion include irregular structures, calcification, cystic or necrotic portion, encasement of mediastinal structure, or an irregular interface with the adjacent lung (6,8). However, the absence of a tissue plane between the neoplasm and adjacent structures does not necessarily signify invasion; thus, the surgeon’s in situ assessment remains the most reliable (7). Drop metastases to the ipsilateral pleural space may manifest as pleural nodules and may progressively coalesce to encase the lung, simulating the radiologic appearance of a diffuse malignant mesothelioma (6). However, in case of extensive pleural involvement by tumor, pleural effusion is rare (9).
Thymic carcinoma
Thymic carcinoma manifests as a large and highly aggressive anterior mediastinal masse in which areas of necrosis, hemorrhage, calcification, or cystic formation may be seen (10). Invasion of the great vessels, lymph node enlargement, phrenic nerve palsy or extrathymic metastases are suggestive of thymic carcinoma rather than thymoma (11).
Thymic carcinoid
Thymic carcinoid has been described as prevascular mediastinal mass that is generally indistinguishable from thymomas and thymic carcinomas at CT (12). About 25% of patients with thymic carcinoids have a positive history of multiple endocrine neoplasia type 1 (MEN-1) (13) and MEN-1 associated thymic carcinoids occurred almost only in male adults.
Teratoma
Teratoma typically manifests as multilocular cystic mass with a thin capsule and internal soft-tissue septa, containing soft-tissue, fluid, fat, or calcium attenuation, or any combination of the four. The combination of fluid, soft-tissue, calcium, and fat attenuation in a cystic prevascular mediastinal mass is a highly specific finding that permits the prospective radiologic diagnosis of mature teratoma (14). High lipid content in the cyst fluid may produce fat-fluid level that is a highly specific finding. Inhomogeneity of the internal components and changes in the adjacent lung parenchyma, pleura, or pericardium can be used as signs of tumor rupture (15).
Malignant germ cell tumors
Seminomas appear large and coarsely lobulated and typically have homogeneous soft-tissue attenuation. They may mimic lymphoma with nodal coalescence. The neoplasm may obliterate tissue planes or directly invade adjacent structures (16). Slight homogeneous enhancement may occur after contrast administration. Rarely, necrosis may result in cystic changes with little residual solid tumor, mimicking a multilocular thymic cyst. Calcification is rare. Metastases to regional lymph nodes and bone occasionally occur. Nonseminomatous malignant germ cell tumors are typically large, with heterogeneous attenuation, and may be well-circumscribed or poorly defined. Extension to both sides of the midline and mass effect are common. CT scan shows extensive central areas of heterogeneous low attenuation due to necrosis and hemorrhage. A border of high attenuation along the periphery of the tumor that enhances after administration of intravenous contrast material and calcification have been described (16). Tissue planes are frequently obliterated. The tumor may invade mediastinal structures, lung, and chest wall and may metastasize to regional lymph nodes and distant sites. Pleural and pericardial effusions are common.
Thymic lymphoma
Thymic lymphoma is a heterogeneous group of lymphoproliferative neoplasm. Common subtypes of thymic lymphoma are primary mediastinal large B-cell lymphoma, precursor T-lymphoblastic lymphoma, and Hodgkin lymphoma. Primary mediastinal large B-cell lymphoma (PMLBCL) most probably arises intrathymically (17) and then aggressively invades adjacent structures and tissues, including regional lymph nodes, whereas distant lymph nodes are rarely affected. Characteristic features of PMLBCL include regular contour and absence of cervical and abdominal lymphadenopathy (18). The presence of vascular involvement is associated with increased likelihood of PMLBCL. Precursor T-lymphoblastic lymphoma (PTLBL) occur most frequently in late childhood, adolescence, and young adulthood, with a male predominance. CT findings including the presence of cervical lymph nodes or inguinal lymph nodes, the presence of pericardial effusion, and the absence of surface lobulation is significantly associated with the likelihood of PTLBL (18). Hodgkin lymphoma (HL) typically spreads to contiguous lymph node regions, rather than showing discontinuous dissemination. Masses typically exhibit homogeneous soft-tissue attenuation, while large tumors may demonstrate heterogeneity with complex low attenuation representing necrosis, hemorrhage, and cystic degeneration. Characteristic features of HL include irregular contour of the anterior mediastinal mass and high prevalence of associated mediastinal lymphadenopathy. CT findings independently associated with increased likelihood of HL were surface lobulation, the absence of vascular involvement, and pleural effusion (18).
Conclusions
A new definition of mediastinal compartments by ITMIG is designed to enable precise identification of mediastinal abnormalities on CT or MRI and helps to make a differential diagnosis. Some mediastinal masses manifest with specific features on CT or MRI which lead to a correct diagnosis with imaging alone.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors Mirella Marino and Brett W. Carter for the series “Dedicated to the 9th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2018)” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2019.04.05). The series “Dedicated to the 9th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2018)” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Carter BW, Tomiyama N, Bhora FY, et al. A modern definition of mediastinal compartments. J Thorac Oncol 2014;9:S97-101. [Crossref] [PubMed]
- Choi YW, McAdams HP, Jeon SC, et al. Idiopathic multilocular thymic cyst: CT features with clinical and histopathologic correlation. AJR Am J Roentgenol 2001;177:881-5. [Crossref] [PubMed]
- Strollo DC, Rosado-de-Christenson ML. Tumors of the thymus. J Thorac Imaging 1999;14:152-71. [Crossref] [PubMed]
- Lattes R. Thymoma and other tumors of the thymus: an analysis of 107 cases. Cancer 1962;15:1224-60. [Crossref]
- Morgenthaler TI, Brown LR, Colby TV, et al. Thymoma. Mayo Clin Proc 1993;68:1110-23. [Crossref] [PubMed]
- Rosado-de-Christenson ML, Galobardes J, Moran CA, et al. Thymoma: radiologic-pathologic correlation. RadioGraphics 1992;12:151-68. [Crossref] [PubMed]
- Kushihashi T, Fujisawa H, Munechika H, et al. Magnetic resonance imaging of thymic epithelial tumors. Crit Rev Diagn Imaging 1996;37:191-259. [PubMed]
- Tomiyama N, Müller NL, Ellis SJ, et al. Invasive and noninvasive thymoma: distinctive CT features. J Comput Assist Tomogr 2001;25:388-93. [Crossref] [PubMed]
- Rosenow EC. m Hurley BT. Disorders of the thymus. Arch Intern Med 1984;144:763-70. [Crossref] [PubMed]
- Do YS, Im JG, Lee BH, et al. CT findings of malignant tumors of thymic epithelium. J Comput Assist tomogr 1995;19:192-7. [Crossref] [PubMed]
- Jung KJ, Lee KS, Han J, et al. Malignant thymic epithelial tumors: CT-pathologic correlation. AJR Am J Roentgenol 2001;176:433-9. [Crossref] [PubMed]
- Quint LE. Imaging of anterior mediastinal masses. Cancer Imaging 2007;7 Spec No A:S56-62.
- Teh BT, Zedenius J, Kytola S, et al. Thymic carcinoids in multiple endocrine neoplasia type 1. Ann Surg 1998;228:99-105. [Crossref] [PubMed]
- Moeller KH, Rosado de Chrisenson ML, Templeton PA. Mediastinal mature teratoma: Imaging features. AJR Am J Roentgenol 1997;169:985-90. [Crossref] [PubMed]
- Choi SJ, Lee JS, Song KS, et al. Mediastinal teratoma: CT differentiation of ruptured and unruptured tumors. AJR Am J Roentgenol 1998;171:591-4. [Crossref] [PubMed]
- Rosado-de-Christenson ML, Templeton PA, Moran CA. Mediastinal germ cell tumors: Radiologic and pathologic correlation. Radiographics 1992;12:1013-30. [Crossref] [PubMed]
- Addis BJ, Isaacson PG. Large cell lymphoma of the mediastinum: a B-cell tumour of probable thymic origin. Histopathology 1986;10:379-90. [Crossref] [PubMed]
- Tateishi U, Müller NL, Johkoh T, et al. Primary mediastinal lymphoma: characteristic features of the various histological subtypes on CT. J Comput Assist Tomogr 2004;28:782-9. [Crossref] [PubMed]
Cite this article as: Tomiyama N. Approach to the prevascular mass. Mediastinum 2019;3:17.


