Radiotherapy for stage IVa thymoma—Shanghai Chest experience
Introduction
Thymomas are neoplasms arising from the thymic epithelial cells. Primary stage IVa thymoma accounts for about 6.8% in all thymic tumor cases (1). Also, pleural recurrence is the most common failure type after curative treatment such as surgical resection (2,3). They are considered a disseminated disease and are usually challenging in terms of treatment approach. By far, the treatment of pleural metastasis is still controversial. Here, we reported our experience in managing stage IVa thymoma, using radiotherapy (RT) as a major modality.
Part 1: intensity modulated radiotherapy (IMRT) for pleural recurrence of thymoma
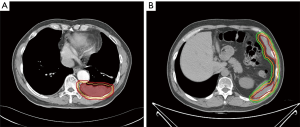
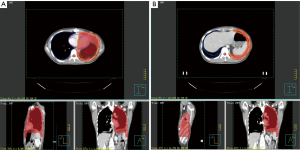
This study was started from 2012, and registered as a phase II clinical trial (ChiCTR-ONC-12002095). It was approved by the Ethical Committee of Shanghai Chest Hospital. Informed consents were collected from all the patients. The inclusion criteria includes: (I) pathologically proven thymoma; (II) measurable pleural lesion on CT image; (III) patients are intolerant of or refuse surgery; (IV) progression after chemotherapy. The target of radiation was contoured according to the following principles: the gross tumor volume (GTV) included all visible tumors; a margin of 4–5 mm was added to GTV to form the clinical tumor volume (CTV). Considering the movement of organs and set-up error, we expanded another 5 mm margin beyond CTV to form the planning tumor volume (PTV). When the pleural lesion was single, it was countered as Figure 1A, whereas for multiple pleural lesions, the target was countered as Figure 1B. There were three dose categories of 30, 40 and 50 Gy. Since all patients had received previous RT to mediastinal area, the total dose of previous RT and current RT was restricted under V20 <35% and MLD <16 Gy.
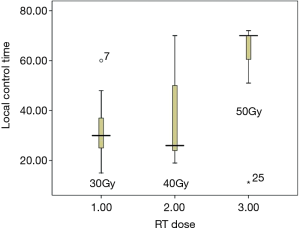
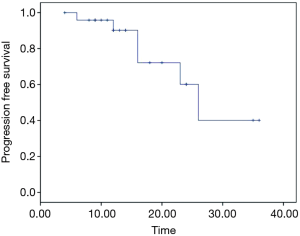
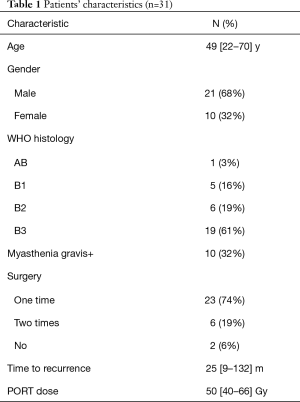
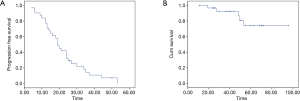
From February 2012 to August 2016, there were totally 31 patients enrolled in this study. The patients’ information was summarized in Table 1. In the median follow-up of 48 [24–70] months, the overall response rate was 97% (CR 45% + PR 52%). The median local control time was 40 (95% CI: 32.6–47.3) months. There were 10 (32%) patients who developed in-field recurrence, while 29 (93.5%) patients developed out-field recurrence. The local control time of different RT dose was shown as Figure 2. The progression free survival (PFS) and overall survival of all patients are shown as Figure 3A,B.
Full table
There were 29 patients who developed out-field recurrence. Twenty-six of them received re-RT; 2 of them received I125 seeds implantation and one received palliative chemotherapy. The toxicity included radiation-induced pneumonitis (7 of grade 3 and 5 of grade 5) and thoracic cavity contracture.
In summary, IMRT is highly effective for pleural recurrence of thymoma, and the ORR is more than 90%. With the increasing dose, the local control rate is improved, however, the incidence of out-field recurrence is still very high. Main toxicity is pneumonitis, and repeated IMRT is associated with higher risk.
Part 2: surgery plus hemithoracic RT
Pleural lesions are often resectable and with postoperative hemithoracic RT, high survival rates are also achievable (4-6). The rationale of using post-operative hemithoracic irradiation is based on three main points: the known sensitivity of such tumors to radiation; the known application of this RT technique in mesotheliomas after extra-pleural pneumonectomy and the fact that the risk of recurrence of the disease is much higher loco-regionally than distantly (7-9).
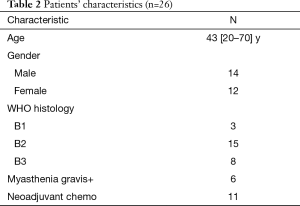
We started our study of surgery plus entire hemithoracic radiotherapy (EHRT) from November 2014. The protocols include: (I) complete resection of all visible tumors; (II) EHRT is started 4–6 weeks after surgery and delivered via IMRT; (III) RT dose is 13 Gy in 13 fractions; (IV) if the T stage is beyond T2, then 30 Gy radiation will be given to mediastinal tumor bed. The target contouring is shown in Figure 4A (above diaphragm level) and Figure 4B (below diaphragm level). By October 2017, we had totally 26 patients enrolled in this study, and patients’ characteristics were summarized in Table 2.
Full table
During a median follow-up time of 19 [12–44] months, 6 (23%) patients developed recurrence. Two of them were in-field and 4 were out-field. The median time to recurrence was 20 [12–26] months. The PFS of the whole group was shown as Figure 5.
Fatigue, nausea and vomiting were the most common side effects, but most of them were mild and easy to relieve. There were also two cases of pyothorax and two cases of myasthenia gravis deterioration, which were recovered by proper management.
In summary, surgery plus EHRT shows good local control with mild toxicity. Since there appeared in-field and out-field recurrence, a more proper radiation field and dose need to be further explored, and the outcome may improve sequentially. Long-term follow-up is needed to check if the advantage of PFS can turn into a longer OS.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors Mirella Marino and Brett W. Carter for the series “Dedicated to the 9th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2018)” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2019.02.01). The series “Dedicated to the 9th International Thymic Malignancy Interest Group Annual Meeting (ITMIG 2018)” was commissioned by the editorial office without any funding or sponsorship. WF serves as an unpaid Editor-in-Chief of Mediastinum. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethical Committee of Shanghai Chest Hospital. Informed consents were collected from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84. [Crossref] [PubMed]
- Kondo K, Monden Y. Lymphogenous and hematogenous metastasis of thymic epithelial tumors. Ann Thorac Surg 2003;76:1859-64. [Crossref] [PubMed]
- Rimner A, Gomez DR, Wu AJ, et al. Failure patterns relative to radiation treatment fields for stage II-IV thymoma. J Thorac Oncol 2014;9:403-9. [Crossref] [PubMed]
- Tagawa T, Kometani T, Yamazaki K, et al. Prognosis and therapeutic response according to the World Health Organization histological classification in advanced thymoma. Surg Today 2011;41:1599-604. [Crossref] [PubMed]
- Sugie C, Shibamoto Y, Ikeya-Hashizume C, et al. Invasive thymoma: postoperative mediastinal irradiation, low-dose entire hemithorax irradiation in patients with pleural dissemination. J Thorac Oncol 2008;3:75-81. [Crossref] [PubMed]
- Huang J, Rizk NP, Travis WD, et al. Feasibility of multimodality therapy including extended resections in stage IVA thymoma. J Thorac Cardiovasc Surg 2007;134:1477-84. [Crossref] [PubMed]
- Hung AY, Eng TY, Scarbrough TJ, et al. CRT. Mediastinum and trachea. In: Halperin EC, Perez CA, Brady LW. editors. Perez and Brady’s principles and practice of radiation oncology. 5th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2008:1109-17.
- Sovak MA, Aisner SC, Aisner J. Tumors of the pleura and mediastinum. In: Abeloff MD, Armitage J, Niederhuber J, et al. editors. Abeloff’s clinical oncology. 4th ed. Philadelphia: Churchill Livingstone/Elsevier, 2008:1367-98.
- Korst RJ, Kansler AL, Christos PJ, et al. Adjuvant radiotherapy for thymic epithelial tumors: a systematic review and meta-analysis. Ann Thorac Surg 2009;87:1641-7. [Crossref] [PubMed]
Cite this article as: Wang C, Gao L, Fang W. Radiotherapy for stage IVa thymoma—Shanghai Chest experience. Mediastinum 2019;3:7.