
The pathology of the thymus in myasthenia gravis
Introduction
Myasthenia gravis (MG) is an autoimmune disease eliciting muscle fatigability and weakness by an autoantibody attack against various proteins that physically and functionally cooperate and stabilize the highly ordered and dense array of acetylcholine receptors (AChRs) on the tips of the postsynaptic folds of the neuromuscular junction as a prerequisite to maintain highly efficient neuromuscular signal transduction (1,2). This “target heterogeneity” of the various autoantibodies is accompanied by somewhat different clinical presentation, different pathogenic mechanisms, different genetic risk factors and different accompanying thymic alterations (3). Currently, MG is classified into:
- MG with anti-AChR autoantibodies (AChR-MG), i.e., the most prevalent MG subtype that occurs in 70–80% MG patients and is due to mainly complement activating IgG1 autoantibodies against the muscle-type AChR (1,4). This subset now includes patients that were up to recently considered as ‘sero-negative’, because their autoantibodies do not bind solubilized AChRs (the targets used in conventional radio-immuno-assay) but recognize AChRs only in its clustered configuration, requiring cell based assays for recognition (5);
- MG with antibodies against the MuSK (MuSK-MG) (6) that is mainly due to IgG4 antibodies that complement-independently interfere with the MuSK/low-density lipoprotein receptor-related protein 4 (LRP4) interaction (7);
- MG due to mainly complement activating IgG1 and IgG2 antibodies to LRP4 (LRP4-MG), an interaction partner of MuSK (8-10);
- MG due to autoantibodies against the motor neuron-derived LRP4 ligand, agrin (Agrin-MG) (11,12) and;
- MG without known target autoantigen(s) (tentatively called “quadruple sero-negative MG”, qSN-MG) (13,14).
In addition, there are small subsets of “double-sero-positive” MG patients with more than one of the above myasthenogenic autoantibodies, such as AChR/LRP4-MG (13,15), AChR/agrin-MG (12,13), MuSK/LRP4-MG (8,16) and, rarely AChR/MuSK MG (17,18). Switches from AChR-MG to AChR/LRP4-MG after thymoma removal (19) and many years after thymectomy for early onset myasthenia gravis (EOMG) (20) have been observed as well.
Another dimension of MG complexity results from the heterogeneity of AChR MG that is subdivided on epidemiological, genetic, clinical and thymic pathological grounds into, thymoma-associated MG (TAMG) and the non-thymomatous subtypes, EOMG, and late onset MG (LOMG). Furthermore, in many patients with AChR-MG various autoimmune targets other than the AChR are attacked by autoantibodies as well, resulting in accompanying diseases that are clinically highly relevant and potentially life-threatening, such as autoimmune thyroid disease, SLE or type I diabetes in EOMG patients; autoimmune pure red cell aplasia, cytopenias, hypogammaglobulinemia (good syndrome), encephalitis and many others in TAMG (21,22). Despite the diametrically different thymic pathologies in TAMG (thymic tumor) and EOMG (thymic ‘atrophy’), there is a surprisingly strong immunological overlap between TAMG and LOMG in terms of the diagnostically important anti-titin autoantibodies and autoantibodies against various cytokines (23,24).
The prevalence of MG among the rare patients with thymolipoma appears to be higher than among members of the healthy population: in a recent study 4.4% of 267 MG-related thymectomies revealed thymolipoma, and stable remission of MG after thymectomy was achieved in a proportion of patients that was similar to that observed in EOMG patients (about 42%) (25). All thymolipoma patients with associated MG (TLAMG) described to date appear to have suffered from AChR-MG. This small subgroup of MG patients has not been studied sufficiently to allow for meaningful speculations in terms of disease mechanisms.
Detailed descriptions of the epidemiological, immunological and clinical heterogeneity of AChR-MG are given in recent reviews (1,2). Interestingly, several epidemiological studies revealed a real increase of LOMG, i.e., AChR-MG in the elderly, for unknown reasons (26,27). Reported incidence rates of AChR-MG varied widely between <1–14 (average 7) per million population (27).
The description of the largely unknown etiologies and the different pathogenetic pathways that lead to the various MG subtypes is beyond the scope of this article and available in recent reviews (1,7,28). Our focus here is on the pathology of the various thymic alterations that have recurrently been encountered in MG patients and are considered to be of pathogenetic relevance (Table 1). We shall also shortly address MG associated with extrathymic tumors. By contrast, we shall not cover thymic carcinomas (TCs). TCs are also derivatives of thymic epithelial cells but resemble carcinomas elsewhere and are labelled as such, e.g., as squamous cell carcinomas. Since TCs usually lack thymic functions (e.g., intratumorous thymopoiesis), associated autoimmunity is rare (e.g., polymyositis) or almost non-existent (e.g., MG). Reports on MG-associated TCs could result from the fact that (I) B3 thymomas were once called “Well differentiated thymic carcinomas” (36) and can be difficult to distinguish from TCs; and (II) myasthenogenic thymoma components accompanying TCs in heterogeneous cancers were not appreciated (37).
Full table
Thymic follicular hyperplasia (TFH)
Thymuses with TFH show increased numbers of lymphoid follicles in the medulla and perivascular spaces. Cortico-medullary architecture is adequate-for-age in corticosteroid-naïve patients. While single lymphoid follicles can occur in healthy persons (38-40), follicles in more than a third of thymic lobules are likely pathological (41).
Many autoimmune diseases can be associated with TFH (3) but TFH is commonest in EOMG, i.e., in patients with non-thymomatous AChR-MG that are mostly less than 50 years of age, but may rarely be up 55 (males) and 65 (females) (29). TFH due to EOMG has an incidence of 1–10 per 1,000,000 per year (42). TFH is also common (30–50%) in remnant thymuses adjacent to thymomas in TAMG and thought to be of a source of autoantibodies (43). By contrast, the reported frequencies of TFH in MuSK-MG (21%), LRP4-MG (0–31%) and AChR(−)/MuSK(−)/LRP4(−) triple-negative MG (22%) (16) need validation. The initiating trigger(s) of TFH are unknown, while many later steps of the intrathymic pathogenesis of EOMG have been resolved, leading to intrathymic autoantibody production (1,43). Since production of autoantibodies in EOMG is higher inside than outside the thymus, the thymus is thought to be the primary site of autoimmunization in EOMG (44), providing the rational for early thymectomy (35).
Clinical considerations: in contrast to thymomas und “true thymic hyperplasia” (45), TFH does not cause local symptoms. Systemic symptoms in TFH result from the underlying autoimmune diseases.
Macroscopy: thymic weight and size are normal or slightly increased for age (38). After corticosteroid treatment, strong thymic shrinkage is common.
Histology and immunohistochemistry: in corticosteroid-naïve patients thymuses show a normal-for-age cortex with terminal deoxynucleotidyl transferase (TdT) + immature T cells, while medullary areas are expanded at sites where lymphoid follicles occur (Figure 1). Follicles show CD21+/CD23+/CD35+ follicular dendritic cell (FDC) networks and may or may not show reactive germinal centers. The number of Hassall corpuscles is normal. Lymphoid follicles disrupt the normally continuous, epithelial network that separates the medulla from epithelial-free perivascular spaces, leading to fusion and expansion of both compartments in which mature B cells and T cells are increased (46). Prolonged TFH can induce thymic epithelial hyperplasia. Corticosteroid treatment leads to a starry sky pattern and shrinkage of the thymic cortex, and collapse of germinal centers and FDC networks. Prolonged, high dose corticosteroid and azathioprine treatment can completely abolish cortical structures and TFH (47), induce shrinkage of the medulla and may efface Hassall’s corpuscles. A grading system of TFH has been proposed (41). Staining of FDC networks for CD21, CD23 or CD35 helps to detect early TFH and remnant follicles after corticosteroid treatment (41).

Differential diagnosis: lymphoid follicles adjacent to TdT+ lymphocyte-rich cortical structures can occur in MG-associated type B1 and B2 thymomas. In small biopsies, thick fibrous capsular structures or medullary islands (MIs) abutting fibrous septae may hint to a B1 thymoma, while increased numbers and clusters of epithelial cells suggest B2 thymoma (48). While ‘pure’ micronodular thymoma (MNT) with lymphoid stroma is usually not associated with MG, type A or AB thymomas with a micronodular component can be accompanied by MG. In small biopsies nodules of spindle epithelial cells can hint to the correct diagnosis. Sampling of remnant thymus with TFH adjacent to an MG-associated thymoma is a pitfall in small biopsies, underlining the necessity to correlate histology with imaging findings. In many other mediastinal diseases associated with lymphoid follicles, MG is typically not present, including mediastinal cysts (if not associated with EOMG or TAMG) (49), ‘pure’ TCs (see above), germ cell tumors, lymphomas, and ‘LESA-like’ thymic hyperplasia (50), showing that information on the MG status helps interpreting small thymic biopsies.
Thymoma
Ten to 20% of MG patients have a thymoma and 30% of thymoma patients have TAMG. TAMG typically occurs after age 40 but may affect children. Thymomas are epithelial tumors that the World Health Organization (WHO) classification (51) subdivides into the malignant A, AB, B1, B2, and B3 rare other types. They usually maintain thymic functions (e.g., intratumorous thymopoiesis). Since this thymopoiesis fails to induce immunological tolerance, thymomas are often associated with autoimmune diseases, the commonest being MG (3). Here we shall cover the potentially MG-associated A, AB, B1, B2 and B3 thymomas (Table 2).
Full table
Type A thymoma, including the ‘atypical type A thymoma’ variant
Type A thymoma is a clinically indolent tumor composed of bland spindle/oval epithelial cells, with few or no admixed immature T cells. The more aggressive atypical variant can display hypercellularity, high mitotic activity and focal necrosis, of which the latter is correlated with increased invasiveness (48). For stage distribution see ref. (51). Since it is a tumor that is lymphocyte-poor or lymphocyte-free throughout, type A thymoma is rarely associated with MG.
Macroscopically, type A thymoma it is usually encapsulated or well circumscribed (stage I or II). The atypical variant may be poorly circumscribed, invade adjacent organs and show metastasis (52-54).
Histologically (Figure 2A,B,C,D), type A thymomas show fascicular, storiform, glandular/adenoid, solid, rosette-forming, hemangiopericytoma-like and paraganglioma-like patterns. Bland spindle or oval cells with small, spindly or oval nuclei with fine chromatin and inconspicuous nucleoli, and thin-walled hemangiopericytomatous vessels are found in almost all cases. Polygonal tumor cells are an optional feature. Lymphoid cells are scarce. Mitoses, apoptotic cells and perivascular spaces are rare. Coagulation necrosis is typical of the atypical variant. Hassall corpuscles are absent.
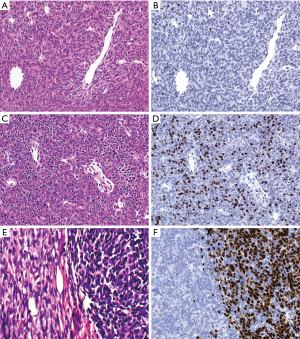
Immunohistochemically, type A thymomas harbor no or rare TdT(+)/CD99(+)/CD1a(+) immature T cells, with TdT being the preferred marker. Any “crowded” immature T cells or moderate numbers of immature T cells in >10% of the evaluable tumor area imply a diagnosis of AB thymoma. Epithelial cells consistently stain for CK19 and p63/p40, are commonly CD20(+) (focally) but negative for CK20, CD5 and CD117. The Ki67 index is <2% in the conventional type A thymoma (55), and may be higher in the atypical variant (own observation).
Genetically, recurrent structural genetic alterations are rare (56), while a unique point mutation in the GTF2I gene (L404H) shows the highest prevalence (80%) among all thymomas (57).
Differential diagnoses comprise other spindle cell ‘lesions’: AB thymoma, focally spindling B3 thymoma, sarcomatoid carcinoma, spindle cell carcinoid; melanoma, synovial sarcoma, solitary fibrous tumor, inflammatory myofibroblastic tumor, nerve sheath tumors, mesothelioma and dendritic cell tumors.
Complete surgical removal is the only definite treatment that may be difficult in ‘atypical’ cases (54). Ten-year survival rates reach 80–100% (58,59) but are unknown for the atypical variant.
Type AB thymoma
AB thymomas are indolent epithelial tumors with a lymphocyte-poor (type A) und lymphocyte-rich (B-like) component in variable proportions. The B-like component must not be subtyped (e.g., as B1-like or B2-like). Stage distribution is given in ref. (51). MG occurs in 20–40% of cases, and other autoimmune diseases are also frequent (22).
Macroscopically, most cases are well circumscribed. Atypical cases that resemble ‘atypical A thymoma’ (e.g., with necrosis, extensive invasion, metastasis) are rare (<5%) (52).
Histologically and immunohistochemically (Figure 2C,D,E,F) type A areas resemble type A thymomas. The B-like areas show spindly, oval or rarely polygonal tumor cells with small nuclei and inconspicuous nucleoli, i.e., they do not look like tumor cells in B thymomas. Light staining ‘MIs’ can occur. Rarely, AB thymomas are immature T-cell-rich throughout, i.e., type A areas and a biphasic pattern are not obligatory for the diagnosis. Spindly and CD20(+) tumor cells are helpful to recognize such cases. In contrast to B1 thymomas, AB thymomas are epithelial-rich throughout on keratin and p63/p40 stains. Focal epithelial CD20 positivity occurs in 50% of cases. Epithelial membrane antigen (EMA) positivity in septal structures is another helpful feature (60). Lymphocytes outside medullar islands are mainly immature, CD3+ TdT(+) T cells with a Ki-67 index >90%.
Genetically, structural alterations are more common than in type A thymomas (56), while the hot spot GTF2I mutation is similarly prevalent (74–80% of cases) (57).
Differential diagnoses comprise A, B1, B2 and MNT with lymphoid stroma. In MNTs the lymphoid component is localized outside the epithelial component. Tumors featuring type A or AB thymoma and an MNT component are common and may be MG associated.
Complete resection is the only curative therapy and usually achieved due to the common low tumor stage. Ten-year overall survival rates are over 80% (58,59).
Type B1 thymoma
B1 thymoma resembles the normal childhood thymus with respect to abundance of immature T cells, paucity of epithelial cells, absence of epithelial cell clustering and the “organoid” concurrence of prevailing cortical areas over minor ‘MIs’. Hassall corpuscles are not obligatory. As to stage distribution, see ref. (51). MG is frequent (−45%), other autoimmune diseases are rare (5%). Pure B1 thymomas usually are indolent tumors (stage I and II in >80%) (58,59).
Macroscopically most B1 thymomas are well circumscribed. The mostly firm capsule confines a soft interior. Tumor nodules are typically large and separated by delicate or coarse fibrous septae.
Histologically and immunohistochemically (Figure 3A,B), B1 thymomas show little or no lobulation. Dark cortical regions dominate over lighter MIs. MIs are, however, not consistently ’buried’ in cortical areas like in pediatric thymuses, but are often misplaced to the periphery of tumor lobules or fibrous septae. Hassall corpuscles occur in 50% of cases and usually not in all MIs in a given case. The abundance of epithelial cells must be similar to that of the normal pediatric thymus and clustering (i.e., 3 or more contiguous tumor cells) must be absent (best appreciated in p40/p63 stains). Tumor cells show vesicular chromatin and variably prominent nucleoli. Immunohistochemical stains show a delicate cytokeratin positive epithelial network that is attenuated in MIs. While CD3(+)TdT(+) T cells dominate in cortical regions, CD3+/TdT(−) T cells prevail over CD20(+) B cells in MIs. Desmin(+) myoid cells and autoimmune regulator [AIRE(+)] epithelial cells occur in (some) MIs in 50% of cases.
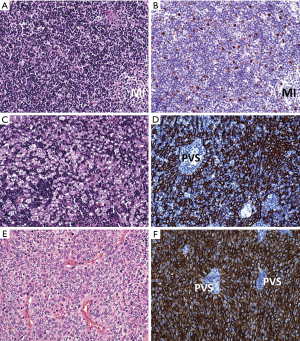
Genetically, chromosomal gains and losses are less frequent than in the more aggressive B2 and B3 thymomas (56). The GTF2I mutation occurs in 32% of cases (57).
Differential diagnoses comprise normal thymus, rebound hyperplasia, true thymic hyperplasia, and T-lymphoblastic lymphoma (T-LBL), all of with are not associated with MG. In small biopsies distinction between normal thymus, TFH and B1 thymoma (with lymphoid follicles) may be impossible. Excess of cortical over medullary areas, misplaced MIs and deficient Hassall corpuscles favor a diagnosis of B1 thymoma. B-like areas of AB thymomas are distinguished by spindle cells and numerous keratin(+) epithelial cells that are CD20(+) in 50% of cases. B2 thymomas show increased numbers or clusters of tumor cells.
Over 90% of B1 thymomas are cured by resection; 10-year and 20-year survival is 85–100% (58,59).
Type B2 thymoma
B2 thymomas are TdT(+) T cell-rich, aggressive tumors with increased numbers of polygonal and dendritic tumor cells compared to B1 thymomas. Spindle tumor cells are absent.
As to stage distribution, see ref. (51). Occurrence in children is rare (61). MG occurs in up to 50% of cases (59), pure red cell aplasia and hypogammaglobulinemia are infrequent (5%). Pleural effusions and the superior vena cava syndrome are commoner than in A, AB and B1 thymomas.
Macroscopically B2 thymomas often infiltrate mediastinal fat, pleura, lung, heart or large vessels. The cut surface is grey-white and firm and may show poor septation, necrosis, haemorrhage and cysts.
Histologically and immunohistochemically (Figure 3C,D), fibrous septae mostly delineate small tumor lobules. Dominant, TdT+ T cells impart a blue impression. Tumor cell nuclei have vesicular chromatin und prominent nucleoli. Perivascular spaces and MIs with or without Hassall corpuscles may occur. Intratumorous lymphoid follicles are common in MG(+) cases. Keratin/p40(+) tumor cells are more numerous than in B1 thymoma and may occur in clusters (3 or more contiguous epithelial cells). Minor B3 or B1 thymoma component occur in 40% of B2 thymomas.
Genetically, the number of alterations in B2 thymomas is intermediate between AB and B3 thymomas (56). 22% of B2 thymomas show the hotspot GTF2I mutation (57).
Differential diagnoses comprise B1 thymomas and T-LBL (see above). Rarely, B2 thymomas show a loss of keratin expression (62). Since expression of p40 is mostly maintained in such cases, a p40 stain is recommended if the differential diagnosis between B2 thymoma and T-LBL is difficult.
Complete resection is achieved in 70–90% of cases and 10-year recurrence rates reach 32% and 41% in R0 resected stage II and III B2 thymomas, respectively. Overall, 10-year survival rates are 70–90% (58,59,63).
Type B3 thymoma
B3 thymomas are epithelial-predominant tumors that show at most a few or no immature, TdT(+) T cells. Tumor cells have largely lost cortical and medullary features (64).
For stage distribution see ref. (51). Occurrence in children is rare (61). MG occurs in 40–50% of cases, other autoimmune diseases are rare. Local symptoms are commoner than in other thymomas.
Macroscopically, B3 thymomas often infiltrate into mediastinal fat and adjacent organs. On the firm or hard, grey/white cut surface, hemorrhage, necrosis and cysts are common.
Histologically and immunohistochemically (Figure 3E,F), tumor lobules are composed of sheets of polygonal tumor cells that impart a pink impression. At the invasion front, lobules are mostly sharply delineated without single cell infiltration that is more common in TC. Tumor cell nuclei are either bland (with inconspicuous nucleoli) or moderately atypical with prominent nucleoli. Among the tumor cells there are usually a few or rarely no lymphocytes that may or may not be TdT(+). Perivascular spaces are often conspicuous. Hassall corpuscles, focal spindle cells and clear cells can occur. There is diffuse and dense expression of keratins and p40/p63; only focal expression of GLUT1 (65) and EMA and no CD20 expression. Focal expression of CD5 and CD117 is rare.
Genetically, B3 thymomas show the highest prevalence of genetic alterations among thymomas (56), while the GTF2I hotspot mutations is as common (21%) as in B2 thymomas (57).
Differential diagnoses: The distinction of B2 thymomas relies on the pink expression of B3 (Figure 3E) versus blue expression of B2 cases (Figure 3C) in HE sections. B3 thymomas with focal spindling and type A thymomas may be impossible to distinguish in small biopsies, if areas with typical features are not sampled (CD20 expression, glandular structures, prominent perivascular spaces). Thymic squamous cell carcinomas (TSQCC) may be difficult to delineate as well, since they can rarely show perivascular spaces and sharply contours at the invasion front. In the presence of MG, a diagnosis of TSQCC should be made with caution and complemented by a search for a thymoma component. Prominent intercellular bridges, expression of CD5 and CD117 (in 80% of TSQCCs), diffuse positivity for GLUT1, and absence of TdT+ T cells suggest a diagnosis of TSQCC (51). Neuroendocrine and germ cell tumors, sarcomas, mesothelioma, and parathyroid adenoma can potentially be misinterpreted as B3 thymoma or TC.
Therapy is like in B2 thymomas. Thirty percent of B3 thymomas recur within 10 years (58). 10-year overall survival rates are 50-70% (59,63). Prognosis of MG(+) cases may be better due to earlier detection (66).
Thymic atrophy
Thymic atrophy has for long been described as the typical ‘pathology’ associated with LOMG, i.e., the non-thymomatous AChR-MG in the elderly [reviewed in ref. (3)]. The lympho-epithelial tissue of the aging thymus is gradually replaced with fat, the cortico-medullary architecture gets distorted (with medullary structures bordering on adipose tissue) (Figure 4A,B), but residual parenchyma continues to export some T-cells at least into middle age (29,67). In LOMG, morphometric analysis of these remnants did not show significant differences between LOMG and normal thymuses (38). Thymic myoid cells are sparse in LOMG (68), decline with age and become barely detectable been 60 and 70 years of age, with considerable variation between patients (69). The number of AIRE positive cells is also declining (own observation), however, again with no clear difference between LOMG and age-matched control thymuses, suggesting that “atrophy” in the LOMG setting may better be labeled as (physiological) “involution”. Nevertheless, there are currently non-histological features of LOMG patients that strongly hint to an involvement of the thymuses in the pathogenesis of LOMG. These features comprise (I) the unique immunological overlap in terms of anti-striational muscle (mostly anti-titin) and anti-cytokine autoantibodies between TAMG (in which an involvement of the neoplastic thymic tissue is certain) and LOMG (23,24); and (II) the observation that the thymus in LOMG patients appears to export significantly less naïve T cells than age-matched controls (29). Understanding these functional peculiaritis of LOMG thymuses might reveal tissue markers that might allow recognition of thymic abnormalities at the immunohistochemical level in the future.

Recently, thymic atrophy has also been mentioned as “pathology” in the context of at least subsets of patients with either MuSK-MG (34,46) or LRP4-MG (13,16). As is the case with LOMG, thymectomy has not generally been beneficial in MuSK-MG (70) and LRP4-MG (2), with few exceptions (71). These observations parallel largely unchanged histology findings in MuSK-MG thymectomy specimens (34,46), while analogous standardized morphological studies in LRP4-MG thymuses are still awaited, as are functional studies (e.g., in terms of thymic T cell export) in MuSK-MG and LRP4-MG patients.
Thymolipoma
Thymolipoma is rare among thymic tumors, accounting for 2–9% of all cases, occurring at any age and without clear gender bias (25). Histologically (Figure 4C,D), the tumors consist of a minor component of thymic tissue that is embedded in a dominant component of mature adipose tissue confined by a fibrous, epithelial-free capsule. The thymic component is often atrophic but may rarely show follicular hyperplasia and exceptionally neoplastic transformation (thymoma or carcinoid) (51,72).
Thymolipoma has repeatedly been found associated with MG while mediastinal/thymic lipoma has not, suggesting that there might be a pathogenetic (i.e., non-fortuitous) link between the thymic component and “thymolipoma-associated MG” (TLAMG) (25,73-75). On the other hand, the reported percentages of MG patients among those who suffer from thymolipoma vary widely (2–50%) in rare reports (73,75,76), making risk prediction a matter of speculation. TLAMG is apparently the commonest autoimmune disease associated with thymolipoma, while hypogammaglobulinemia (good syndrome) (77), erythropoietic hypoplasia (78), aplastic anemia, polymyositis (79) and Graves’ disease have been reported less commonly (73). The latter rare autoimmune diseases in TLAMG patients suggest a similarity between TLAMG and TAMG, but there are no immunological data (e.g., in terms of anti-titin or anti-cytokine autoantibodies) to underpin this hypothesis. Finally, no consistent histological alteration (e.g., follicular hyperplasia or atrophy) has been reported in thymolipomas accompanied by TLAMG (80).
Concurrences of MG with other cancers
Extrathymic epithelial, mesenchymal and hematological cancers have rarely been reported in connection with MG, likely reflecting a fortuitous coincidence or the result of unspecific immune perturbation (81,82). However, in a rare MG-associated rhabdomyosarcoma (RMS) an autoimmunizing ‘accident’ against AChRs might have occurred (83). The reason for the ‘accident’ in this unique RMS remained enigmatic, and cannot be explained by expression of the AChR, because almost all RMS express functional AChRs (84). MG-associated non-thymic cancers may deserve more attention as exemplified by rare AChR-MG-associated FDC sarcomas that occurred in the mediastinum (85) and mesentery (86). Both cases were the first non-thymic epithelial tumors described to exhibited “thymoma-like” intratumorous thymopoiesis, suggesting that this feature contributed to paraneoplastic MG.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor Mirella Marino for the series “Diagnostic Problems in Anterior Mediastinum Lesions” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2018.12.04). The series “Diagnostic Problems in Anterior Mediastinum Lesions” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Romi F, Hong Y, Gilhus NE. Pathophysiology and immunological profile of myasthenia gravis and its subgroups. Curr Opin Immunol 2017;49:9-13. [Crossref] [PubMed]
- Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol 2015;14:1023-36. [Crossref] [PubMed]
- Marx A, Pfister F, Schalke B, et al. The different roles of the thymus in the pathogenesis of the various myasthenia gravis subtypes. Autoimmun Rev 2013;12:875-84. [Crossref] [PubMed]
- Patrick J, Lindstrom J. Autoimmune response to acetylcholine receptor. Science 1973;180:871-2. [Crossref] [PubMed]
- Leite MI, Jacob S, Viegas S, et al. IgG1 antibodies to acetylcholine receptors in 'seronegative' myasthenia gravis. Brain 2008;131:1940-52. [Crossref] [PubMed]
- Hoch W, McConville J, Helms S, et al. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med 2001;7:365-8. [Crossref] [PubMed]
- Huijbers MG, Zhang W, Klooster R, et al. MuSK IgG4 autoantibodies cause myasthenia gravis by inhibiting binding between MuSK and Lrp4. Proc Natl Acad Sci U S A 2013;110:20783-8. [Crossref] [PubMed]
- Zhang B, Tzartos JS, Belimezi M, et al. Autoantibodies to lipoprotein-related protein 4 in patients with double-seronegative myasthenia gravis. Arch Neurol 2012;69:445-51. [Crossref] [PubMed]
- Pevzner A, Schoser B, Peters K, et al. Anti-LRP4 autoantibodies in AChR- and MuSK-antibody-negative myasthenia gravis. J Neurol 2012;259:427-35. [Crossref] [PubMed]
- Higuchi O, Hamuro J, Motomura M, et al. Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Ann Neurol 2011;69:418-22. [Crossref] [PubMed]
- Gasperi C, Melms A, Schoser B, et al. Anti-agrin autoantibodies in myasthenia gravis. Neurology 2014;82:1976-83. [Crossref] [PubMed]
- Zhang B, Shen C, Bealmear B, et al. Autoantibodies to agrin in myasthenia gravis patients. PLoS One 2014;9:e91816 [Crossref] [PubMed]
- Cordts I, Bodart N, Hartmann K, et al. Screening for lipoprotein receptor-related protein 4-, agrin-, and titin-antibodies and exploring the autoimmune spectrum in myasthenia gravis. J Neurol 2017;264:1193-203. [Crossref] [PubMed]
- Zisimopoulou P, Brenner T, Trakas N, et al. Serological diagnostics in myasthenia gravis based on novel assays and recently identified antigens. Autoimmun Rev 2013;12:924-30. [Crossref] [PubMed]
- Ishikawa H, Taniguchi A, Ii Y, et al. Double-seropositive myasthenia gravis with acetylcholine receptor and low-density lipoprotein receptor-related protein 4 antibodies associated with invasive thymoma. Neuromuscul Disord 2017;27:914-7. [Crossref] [PubMed]
- Zisimopoulou P, Evangelakou P, Tzartos J, et al. A comprehensive analysis of the epidemiology and clinical characteristics of anti-LRP4 in myasthenia gravis. J Autoimmun 2014;52:139-45. [Crossref] [PubMed]
- Hong Y, Zisimopoulou P, Trakas N, et al. Multiple antibody detection in 'seronegative' myasthenia gravis patients. Eur J Neurol 2017;24:844-50. [Crossref] [PubMed]
- Zouvelou V, Kyriazi S, Rentzos M, et al. Double-seropositive myasthenia gravis. Muscle Nerve 2013;47:465-6. [Crossref] [PubMed]
- Jordan B, Schilling S, Zierz S. Switch to double positive late onset MuSK myasthenia gravis following thymomectomy in paraneoplastic AChR antibody positive myasthenia gravis. J Neurol 2016;263:174-6. [Crossref] [PubMed]
- Zouvelou V, Zisimopoulou P, Psimenou E, et al. AChR-myasthenia gravis switching to double-seropositive several years after the onset. J Neuroimmunol 2014;267:111-2. [Crossref] [PubMed]
- Marx A, Porubsky S, Belharazem D, et al. Thymoma related myasthenia gravis in humans and potential animal models. Exp Neurol 2015;270:55-65. [Crossref] [PubMed]
- Marx A, Willcox N, Leite MI, et al. Thymoma and paraneoplastic myasthenia gravis. Autoimmunity 2010;43:413-27. [Crossref] [PubMed]
- Kisand K, Lilic D, Casanova JL, et al. Mucocutaneous candidiasis and autoimmunity against cytokines in APECED and thymoma patients: clinical and pathogenetic implications. Eur J Immunol 2011;41:1517-27. [Crossref] [PubMed]
- Meager A, Wadhwa M, Dilger P, et al. Anti-cytokine autoantibodies in autoimmunity: preponderance of neutralizing autoantibodies against interferon-alpha, interferon-omega and interleukin-12 in patients with thymoma and/or myasthenia gravis. Clin Exp Immunol 2003;132:128-36. [Crossref] [PubMed]
- Huang CS, Li WY, Lee PC, et al. Analysis of outcomes following surgical treatment of thymolipomatous myasthenia gravis: comparison with thymomatous and non-thymomatous myasthenia gravis. Interact Cardiovasc Thorac Surg 2014;18:475-81. [Crossref] [PubMed]
- Carr AS, Cardwell CR, McCarron PO, et al. A systematic review of population based epidemiological studies in Myasthenia Gravis. BMC Neurol 2010;10:46. [Crossref] [PubMed]
- Heldal AT, Eide GE, Gilhus NE, et al. Geographical distribution of a seropositive myasthenia gravis population. Muscle Nerve 2012;45:815-9. [Crossref] [PubMed]
- Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classifications - Authors' reply. Lancet Neurol 2016;15:357-8. [Crossref] [PubMed]
- Chuang WY, Strobel P, Bohlender-Willke AL, et al. Late-onset myasthenia gravis - CTLA4(low) genotype association and low-for-age thymic output of naive T cells. J Autoimmun 2014;52:122-9. [Crossref] [PubMed]
- Saka E, Topcuoglu MA, Akkaya B, et al. Thymus changes in anti-MuSK-positive and -negative myasthenia gravis. Neurology 2005;65:782-3; author reply -3.
- Rigamonti A, Lauria G, Piamarta F, et al. Thymoma-associated myasthenia gravis without acetylcholine receptor antibodies. J Neurol Sci 2011;302:112-3. [Crossref] [PubMed]
- Maggi L, Andreetta F, Antozzi C, et al. Two cases of thymoma-associated myasthenia gravis without antibodies to the acetylcholine receptor. Neuromuscul Disord 2008;18:678-80. [Crossref] [PubMed]
- Leite MI, Strobel P, Jones M, et al. Fewer thymic changes in MuSK antibody-positive than in MuSK antibody-negative MG. Ann Neurol 2005;57:444-8. [Crossref] [PubMed]
- Lauriola L, Ranelletti F, Maggiano N, et al. Thymus changes in anti-MuSK-positive and -negative myasthenia gravis. Neurology 2005;64:536-8. [Crossref] [PubMed]
- Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med 2016;375:511-22. [Crossref] [PubMed]
- Kirchner T, Schalke B, Buchwald J, et al. Well-differentiated thymic carcinoma. An organotypical low-grade carcinoma with relationship to cortical thymoma. Am J Surg Pathol 1992;16:1153-69. [Crossref] [PubMed]
- Kim SH, Koh IS, Minn YK. Pathologic Finding of Thymic Carcinoma Accompanied by Myasthenia Gravis. J Clin Neurol 2015;11:372-5. [Crossref] [PubMed]
- Strobel P, Moritz R, Leite MI, et al. The ageing and myasthenic thymus: a morphometric study validating a standard procedure in the histological workup of thymic specimens. J Neuroimmunol 2008;201-202:64-73. [Crossref] [PubMed]
- Middleton G, Schoch EM. The prevalence of human thymic lymphoid follicles is lower in suicides. Virchows Arch 2000;436:127-30. [Crossref] [PubMed]
- Okabe H. Thymic lymph follicles; a histopathological study of 1,356 autopsy cases. Acta Pathol Jpn 1966;16:109-30. [PubMed]
- Marx A, Pfister F, Schalke B, et al. Thymus pathology observed in the MGTX trial. Ann N Y Acad Sci 2012;1275:92-100. [Crossref] [PubMed]
- Meyer A, Levy Y. Geoepidemiology of myasthenia gravis Autoimmun Rev 2010;9:A383-6. [corrected]. [Crossref] [PubMed]
- Vincent A, Scadding GK, Thomas HC, et al. In-vitro synthesis of anti-acetylcholine-receptor antibody by thymic lymphocytes in myasthenia gravis. Lancet 1978;1:305-7. [Crossref] [PubMed]
- Hohlfeld R, Wekerle H. Reflections on the “intrathymic pathogenesis” of myasthenia gravis. J Neuroimmunol 2008;201-202:21-7. [Crossref] [PubMed]
- Weis CA, Markl B, Schuster T, et al. True thymic hyperplasia: Differential diagnosis of thymic mass lesions in neonates and children. Pathologe 2017;38:286-93. [Crossref] [PubMed]
- Leite MI, Jones M, Strobel P, et al. Myasthenia gravis thymus: complement vulnerability of epithelial and myoid cells, complement attack on them, and correlations with autoantibody status. Am J Pathol 2007;171:893-905. [Crossref] [PubMed]
- Schalke BC, Mertens HG, Kirchner T, et al. Long-term treatment with azathioprine abolishes thymic lymphoid follicular hyperplasia in myasthenia gravis. Lancet 1987;2:682. [Crossref] [PubMed]
- Marx A, Chan JK, Coindre JM, et al. The 2015 World Health Organization Classification of Tumors of the Thymus: Continuity and Changes. J Thorac Oncol 2015;10:1383-95. [Crossref] [PubMed]
- Suster S, Rosai J. Multilocular thymic cyst: an acquired reactive process. Study of 18 cases. Am J Surg Pathol 1991;15:388-98. [Crossref] [PubMed]
- Weissferdt A, Moran CA. Thymic hyperplasia with lymphoepithelial sialadenitis (LESA)-like features: a clinicopathologic and immunohistochemical study of 4 cases. Am J Clin Pathol 2012;138:816-22. [Crossref] [PubMed]
- WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed. Lyon: IARC; 2015.
- Green AC, Marx A, Strobel P, et al. Type A and AB thymomas: histological features associated with increased stage. Histopathology 2015;66:884-91. [Crossref] [PubMed]
- Nonaka D, Rosai J. Is there a spectrum of cytologic atypia in type a thymomas analogous to that seen in type B thymomas? A pilot study of 13 cases. Am J Surg Pathol 2012;36:889-94. [Crossref] [PubMed]
- Jain RK, Mehta RJ, Henley JD, et al. WHO types A and AB thymomas: not always benign. Mod Pathol 2010;23:1641-9. [Crossref] [PubMed]
- Roden AC, Yi ES, Jenkins SM, et al. Diagnostic significance of cell kinetic parameters in World Health Organization type A and B3 thymomas and thymic carcinomas. Hum Pathol 2015;46:17-25. [Crossref] [PubMed]
- Rajan A, Girard N, Marx A. State of the art of genetic alterations in thymic epithelial tumors. J Thorac Oncol 2014;9:S131-6. [Crossref] [PubMed]
- Petrini I, Meltzer PS, Kim IK, et al. A specific missense mutation in GTF2I occurs at high frequency in thymic epithelial tumors. Nat Genet 2014;46:844-9. [Crossref] [PubMed]
- Weis CA, Yao X, Deng Y, et al. The impact of thymoma histotype on prognosis in a worldwide database. J Thorac Oncol 2015;10:367-72. [Crossref] [PubMed]
- Strobel P, Bauer A, Puppe B, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol 2004;22:1501-9. [Crossref] [PubMed]
- Weissferdt A, Hernandez JC, Kalhor N, et al. Spindle cell thymomas: an immunohistochemical study of 30 cases. Appl Immunohistochem Mol Morphol 2011;19:329-35. [Crossref] [PubMed]
- Stachowicz-Stencel T, Orbach D, Brecht I, et al. Thymoma and thymic carcinoma in children and adolescents: a report from the European Cooperative Study Group for Pediatric Rare Tumors (EXPeRT). Eur J Cancer 2015;51:2444-52. [Crossref] [PubMed]
- Adam P, Hakroush S, Hofmann I, et al. Thymoma with loss of keratin expression (and giant cells): a potential diagnostic pitfall. Virchows Arch 2014;465:313-20. [Crossref] [PubMed]
- Okumura M, Ohta M, Tateyama H, et al. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: a clinical study of 273 patients. Cancer 2002;94:624-32. [Crossref] [PubMed]
- Strobel P, Hartmann E, Rosenwald A, et al. Corticomedullary differentiation and maturational arrest in thymomas. Histopathology 2014;64:557-66. [Crossref] [PubMed]
- Thomas de Montpreville V, Quilhot P, Chalabreysse L, et al. Glut-1 intensity and pattern of expression in thymic epithelial tumors are predictive of WHO subtypes. Pathol Res Pract 2015;211:996-1002. [Crossref] [PubMed]
- de Jong WK, Blaauwgeers JL, Schaapveld M, et al. Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur J Cancer 2008;44:123-30. [Crossref] [PubMed]
- Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature 1998;396:690-5. [Crossref] [PubMed]
- Van de Velde RL, Friedman NB. Thymic myoid cells and myasthenia gravis. Am J Pathol 1970;59:347-68. [PubMed]
- Roxanis I, Micklem K, McConville J, et al. Thymic myoid cells and germinal center formation in myasthenia gravis; possible roles in pathogenesis. J Neuroimmunol 2002;125:185-97. [Crossref] [PubMed]
- Guptill JT, Sanders DB, Evoli A. Anti-MuSK antibody myasthenia gravis: clinical findings and response to treatment in two large cohorts. Muscle Nerve 2011;44:36-40. [Crossref] [PubMed]
- Corda D, Deiana GA, Mulargia M, et al. Familial autoimmune MuSK positive myasthenia gravis. J Neurol 2011;258:1559-60. [Crossref] [PubMed]
- Rieker RJ. Thymolipoma. In: WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed. Travis WD, Brambilla E, Burke AP, et al. editors. WHO Classification of Lyon: IARC;2015:289.
- Damadoglu E, Salturk C, Takir HB, et al. Mediastinal thymolipoma: an analysis of 10 cases. Respirology 2007;12:924-7. [Crossref] [PubMed]
- Rieker RJ, Schirmacher P, Schnabel PA, et al. Thymolipoma. A report of nine cases, with emphasis on its association with myasthenia gravis. Surg Today 2010;40:132-6. [Crossref] [PubMed]
- Moran CA, Rosado-de-Christenson M, Suster S. Thymolipoma: clinicopathologic review of 33 cases. Mod Pathol 1995;8:741-4. [PubMed]
- Reintgen D, Fetter BF, Roses A, et al. Thymolipoma in association with myasthenia gravis. Arch Pathol Lab Med 1978;102:463-6. [PubMed]
- Otto HF, Loning T, Lachenmayer L, et al. Thymolipoma in association with myasthenia gravis. Cancer 1982;50:1623-8. [Crossref] [PubMed]
- Saux MC, Mosser J, Pontagnier H, et al. Pharmacokinetics of doxycycline polyphosphate after oral multiple dosing in humans. Eur J Drug Metab Pharmacokinet 1982;7:123-30. [Crossref] [PubMed]
- Santos E, Coutinho E, Martins da Silva A, et al. Inflammatory myopathy associated with myasthenia gravis with and without thymic pathology: Report of four cases and literature review. Autoimmun Rev 2017;16:644-9. [Crossref] [PubMed]
- Le Marc'hadour F, Pinel N, Pasquier B, et al. Thymolipoma in association with myasthenia gravis. Am J Surg Pathol 1991;15:802-9. [Crossref] [PubMed]
- Roche JC, Capablo JL, Ara JR. Myasthenia gravis in association with extrathymic neoplasia. Neurologia 2014;29:507-9. [Crossref] [PubMed]
- Rezania K, Soliven B, Baron J, et al. Myasthenia gravis, an autoimmune manifestation of lymphoma and lymphoproliferative disorders: case reports and review of literature. Leuk Lymphoma 2012;53:371-80. [Crossref] [PubMed]
- Mehmood QU, Shaktawat SS, Parikh O. Case of rhabdomyosarcoma presenting with myasthenia gravis. J Clin Oncol 2011;29:e653-5. [Crossref] [PubMed]
- Gattenlohner S, Marx A, Markfort B, et al. Rhabdomyosarcoma lysis by T cells expressing a human autoantibody-based chimeric receptor targeting the fetal acetylcholine receptor. Cancer Res 2006;66:24-8. [Crossref] [PubMed]
- Hartert M, Strobel P, Dahm M, et al. A follicular dendritic cell sarcoma of the mediastinum with immature T cells and association with myasthenia gravis. Am J Surg Pathol 2010;34:742-5. [PubMed]
- Kim WY, Kim H, Jeon YK, et al. Follicular dendritic cell sarcoma with immature T-cell proliferation. Hum Pathol 2010;41:129-33. [Crossref] [PubMed]
Cite this article as: Marx A, Ströbel P, Weis CA. The pathology of the thymus in myasthenia gravis. Mediastinum 2018;2:66.


