
Investigating thymic epithelial tumor, the “source” of autoimmunity and immunodeficiency: a lesson from ITMIGRD
The International Thymic Malignancy Interest Group Retrospective database (ITMIGRD) is a precious source of data concerning thymic epithelial tumors (TETs), one of the rarest adult malignancies. Thymic neoplasms are a strange type of cancer as concerns biological and clinical behavior and the peculiar association with paraneoplastic syndromes, either autoimmune diseases or clinical manifestations of immunodeficiency. The identification of these immunological disorders is important for physicians that treat these patients.
Among TETs, thymoma is the most predominant, characterized by a unique association with autoimmune diseases, followed by thymic carcinoma, which is less common but more clinically aggressive.
Normal thymic architecture is essential for the proper development of T-lymphocytes. In thymic microenvironment, positive and negative selection of competent not autoreactive cells realize through interactions of immature T-cell progenitors with cortical and medullary thymic epithelial cells (TECs).
This process requires normal thymic architecture, expression of major histocompatibility complex (MHC) class II, and normal expression of the autoimmune regulator (AIRE) gene. On the contrary, thymomas harbor a deranged tumor microenvironment where an abnormal thymopoiesis occurs.
Several abnormalities have been described in thymomas that may affect normal T-cell development: the tumor architecture is distorted, neoplastic cells express less MHC class II, most thymomas do not express AIRE, and production of T-regulator cells is decreased.
Thymomas are associated with a variety of autoimmune disorders often linked to T-cell-mediated autoimmunity (1). Most common autoimmune diseases associated with TETs are myasthenia gravis (MG), Hashimoto’s thyroiditis, Graves’ disease, pure red cell aplasia, aplastic anemia, autoimmune hemolytic anemia, systemic lupus, lichen planus pemphigus and other yet (2). Figures 1 and 2 describe from a clinical and pathological point of view a skin disorder associated with thymoma. Physicians dealing with TETS should keep in mind that usual as well as unusual syndromes are associated with this tumor.
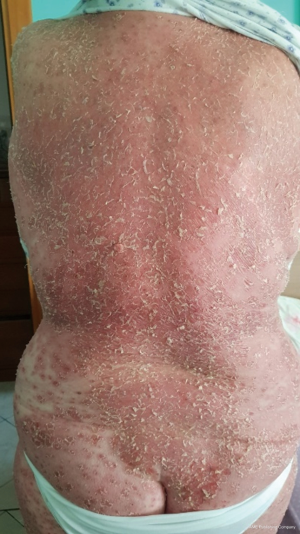
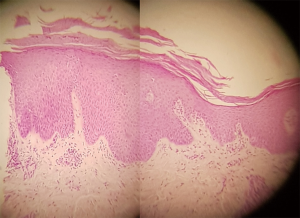
TETs are also associated sometimes with immunodeficiency, which may be latent or clinically evident because of manifestations such as pneumonitis, encephalitis, which are difficult to be managed.
Good’s syndrome (GS) is a thymoma-associated immunodeficiency characterized by hypogammaglobulinemia and a decrease in B lymphocyte described in 1954 by Dr. Good (3). Due to its rarity, GS has been purely investigated and immunological features, as well as pathogenetic mechanisms underlining this syndrome, are now unclear (4). In the first studies of a series of 30 thymoma patients by performing an immunological assessment, including immunophenotype and analysis of T cell repertoire (TCR), a progressive decrease in B, CD4 T and NK lymphocytes, and accumulation of CD8−CD45 RA+ T lymphocytes was showed (5-7). GS is a complex syndrome including a marked loss of CD4+ T lymphocytes and NK cells accompanied by accumulation of polyclonal CD8+ T cells showing the typical phenotype of naive lymphocytes (6). Recently other series of patients with GS are published that confirm the first data (8-12).
Very often autoimmune syndromes and immunodeficiency present together (8,9), thus, making clinical management even more difficult.
The paper from ITMIGRD derives data from 6,670 patients with recognized paraneoplastic/autoimmune (PN/AI) syndromes evaluated over more than 60 years (13).
Most patients (97%) were enrolled from 1991 to 2012, namely about twenty years. A total of 6,297 patients were included in the analysis, but information on recurrence and survival information were available respectively for 4,375 and 4,962 patients. This means that 30% and 22% of the information concerning recurrence and overall survival respectively are missing.
In the analysis by ITMIGRD, PN/AI syndromes were associated with younger age, female sex, thymoma histological type, earlier stage and an increased rate of total thymectomy and complete resection status. These associations reflect common findings: (I) MG, which is the most common PN/AI syndrome associated with TETs, is more frequent in females; (II) thymomas more frequently than TETs are associated with paraneoplastic syndromes; (III) manifestation of syndrome helps in an earlier detection of TET and therefore in a timely intervention which translates into earlier stages and curative surgery. It is not surprising that this early diagnosis makes possible a significant improvement in overall survival in the group with associated syndromes. MG is highly invaliding and significantly worse quality of life of affected patients, especially women as results in a study of 1,315 subjects (14). In this study, the differences between male and female sex were even out after thymectomy, confirming that surgery is beneficial also as concerns quality of life.
The high incidence of thymomas with favorable histology and the reduced number of thymic carcinomas in the group with PN/AI syndrome contribute to better OS.
The improved survival in advanced thymoma with PN/AI syndrome may be related with a better biology or a selection bias, as the authors suggested. Intuitively, given the long period of retrospective observation, an analysis by decade could be useful. In fact, the authors have evaluated separately the patients treated from 1991 to 2012, as compared to the previous years, which were predominant in the series, and found an improved OS when PN/AI is present, while no difference was found in patients without PN/AI over time. The authors suppose that an improved treatment of thymoma-associated syndrome conditions the improved survival found in patients with PN/AI. The long period of evaluation implies changes in classification, treatment and laboratory tests. Nowadays most specialized centers perform an immunological profile, which better characterizes each clinical thymoma-associated syndromes. Moreover, insights into PN/AI have also changed diagnosis and treatment. For example, the new panel of MG autoantibodies includes not only the historical autoantibodies against acetylcholine receptor, but also antibodies directed against the muscle-specific kinase (MUSK), lipoprotein-related protein 4 (LRP4), or agrin in the postsynaptic membrane at the neuromuscular junction discovered (15). New sensitive assays and tailored treatment strategies are also available in MG (16).
TETs include tumors that are different in several respects: thymic carcinomas and thymic neuroendocrine tumors (TNT) are different from thymomas. For example, TNTs occur more in male than female sex, 5- and 10-year survival rates of thymic carcinoids are 56% and 26%, which are significantly worse than thymoma and female sex had a significantly prognostic negative value in surgically treated patients (17). Two analysis from European Society of Thoracic Surgeons and the ITMIGRD showed that thymic carcinomas and neuroendocrine thymic tumors showed instead similar survival rates, while TNTs had worse outcomes (18,19). MG occurs less frequently in TNTs (20,21) as compares to thymoma (30% of patients with thymoma may present MG) (13). In the study by ITMGRD, 6% of the patients with thymic carcinomas had a PN/AI syndrome. Probably further analysis should separate thymomas from thymic carcinomas and TNTs, even if in the study by Padda et al. (13) TNTs represents only 2% and thymic carcinomas are 12%.
The OS improvement in the group with PN/AI syndrome suggests another consideration. Both thymoma and autoimmune syndromes benefit from steroids. As most patients with PN/AI syndrome are treated with steroids, is there a direct role of steroids in tumor dormancy (i.e., prevention of micrometastasis growth)? Data from the ITMIGR database concerning pharmacological and especially steroid treatment could disclose other aspects of this matter.
It should be useful to obtain information on the impact if any of palliative chemotherapy in patients with or without PN/AI syndromes. Data from ITMIGRD (8) showed that both the groups do not receive chemotherapy probably because early Masaoka stages were most represented and total thymectomy was performed in the majority of patients. For the same reasons probably, an increased number of patients with PN/AI syndromes does not receive chemotherapy. Curative chemotherapy is considered an unfavorable prognostic factor in the multivariate analysis. The Authors suggest that this may be due to long-term toxicities. We suppose that other factors may be implicated: (I) unfavorable histology and advanced Masaoka stages, which by themselves, have a negative prognostic value, and are more frequently treated with chemotherapy; (II) low activity and efficacy of standard chemotherapeutic regimens in TETs. Differently from chemotherapy, radiotherapy was an independent favorable prognostic factor for both RFS and OS, probably because radiotherapy was used in less advanced stages.
The authors recognize that some limitations influence the study data, such as the different kind of follow-up performed in different times and different institutions, the missing data as concerns cause of death and the short median follow-up of the group (<4 years). However, the limitations are intrinsic in the retrospective nature of the study and the multi-institutional collection of data. Relevant is the expertise of reference center because it’s only the experience that allows an early diagnosis and appropriate therapy. Guidelines should have been drafted for the most common and rare syndromes associated with TET. The data presented of ITMIG database are inadequate to explain the role of the complex scenario of PN/AI associated with TET tumors. On the other hand, until a preferred prospective study concerning this rare tumor and PN/AI will start, this analysis offers an interesting starting point.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Dr. Luigi Ventura (Thoracic Surgery, Surgical Unit, Department of Medicine and Surgery, University Hospital of Parma, Parma, Italy).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2018.10.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Weksler B, Lu BJ. Alterations of the immune system in thymic malignancies. J Thorac Oncol 2014;9:S137-42. [Crossref] [PubMed]
- Bernard C, Frih H, Pasquet F, et al. Thymoma associated with autoimmune diseases: 85 cases and literature review. Autoimmun Rev 2016;15:82-92. [Crossref] [PubMed]
- Good RA. Agammaglobulinaemia—a provocative experiment of nature. Bulletin of the University of Minnesota 1954;26:1-19.
- Kelesidis T, Yang O. Good's syndrome remains a mystery after 55 years: A systematic review of the scientific evidence. Clin Immunol 2010;135:347-63. [Crossref] [PubMed]
- Vitiello L, Masci AM, Montella L, et al. Thymoma-associated immunodeficiency: a syndrome characterized by severe alterations in NK, T and B-cells and progressive increase in naïve CD8+ T Cells. Int J Immunopathol Pharmacol 2010;23:307-16. [Crossref] [PubMed]
- Masci AM, Palmieri G, Vitiello L, et al. Clonal expansion of CD8+ BV8 T lymphocytes in bone marrow characterizes thymoma-associated B lymphopenia. Blood 2003;101:3106-8. [Crossref] [PubMed]
- Montella L, Masci AM, Merkabaoui G, et al. B-cell lymphopenia and hypogammaglobulinemia in thymoma patients. Ann Hematol 2003;82:343-7. [Crossref] [PubMed]
- Malphettes M, Gérard L, Galicier L, et al. Deficit Immunitaire de l'adulte Study Group Good syndrome: an adult-onset immunodeficiency remarkable for its high incidence of invasive infections and autoimmune complications. Clin Infect Dis 2015;61:e13-9. [Crossref] [PubMed]
- Arnold SJ, Hodgson T, Misbah SA, et al. Three difficult cases: the challenge of autoimmunity, immunodeficiency and recurrent infections in patients with Good syndrome. Br J Dermatol 2015;172:774-7. [Crossref] [PubMed]
- Tavakol M, Mahdaviani SA, Ghaemi MR, et al. Good’s Syndrome-Association of the Late Onset Combined Immunodeficiency with Thymoma: Review of Literature and Case Report. Iran J Allergy Asthma Immunol 2018;17:85-93. [PubMed]
- Zaman M, Huissoon A, Buckland M, et al. Clinical and laboratory features of seventy-eight UK patients with Good's syndrome (thymoma and hypogammaglobulinaemia). Clin Exp Immunol 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Chen X, Zhang JX, Shang WW, et al. Aberrant Peripheral Immune Function in a Good Syndrome Patient. J Immunol Res 2018;2018:6212410 [Crossref] [PubMed]
- Padda SK, Yao X, Antonicelli A, et al. Paraneoplastic Syndromes and Thymic Malignancies: An Examination of the International Thymic Malignancy Interest Group Retrospective Database. J Thorac Oncol 2018;13:436-46. [Crossref] [PubMed]
- Lee I, Kaminski HJ, Xin H, et al. Gender and quality of life in myasthenia gravis patients from the myasthenia gravis foundation of America registry. Muscle Nerve 2018;21:90-8.. [Crossref] [PubMed]
- Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol 2015;14:1023-36. [Crossref] [PubMed]
- Evoli A, Iorio R, Bartoccioni E. Overcoming challenges in the diagnosis and treatment of myasthenia gravis. Expert Rev Clin Immunol 2016;12:157-68. [Crossref] [PubMed]
- Bian D, Qi M, Hu J, et al. The comparison of predictive factors regarding prognoses and invasion of thymic neuroendocrine tumors preoperatively and postoperatively. J Thorac Dis 2018;10:1657-69. [Crossref] [PubMed]
- Filosso PL, Yao X, Ruffini E, et al. Comparison of outcomes between neuroendocrine thymic tumours and other subtypes of thymic carcinomas: a joint analysis of the European Society of Thoracic Surgeons and the International Thymic Malignancy Interest Group. Eur J Cardiothorac Surg 2016;50:766-71. [Crossref] [PubMed]
- Filosso PL, Yao X, Ahmad U, et al. Outcome of primary neuroendocrine tumors of the thymus: a joint analysis of the International Thymic Malignancy Interest Group and the European Society of Thoracic Surgeons databases. J Thorac Cardiovasc Surg 2015;149:103-9.e2. [Crossref] [PubMed]
- Fukai I, Masaoka A, Fujii Y, et al. Thymic Neuroendocrine Tumor (Thymic Carcinoid): A Clinicopathologic Study in 15 Patients. Ann Thorac Surg 1999;67:208-11. [Crossref] [PubMed]
- Girard N. Neuroendocrine tumors of the thymus: the oncologist point of view J Thorac Dis 2017;9:S1491-500. [Crossref] [PubMed]
Cite this article as: Montella L, Ottaviano M, Palmieri G. Investigating thymic epithelial tumor, the “source” of autoimmunity and immunodeficiency: a lesson from ITMIGRD. Mediastinum 2018;2:62.


