Initial experience with a combined sequential left-sided and subxiphoid video-assisted thoracic surgery approach for resection of large anterior mediastinal tumors
Introduction
In 2016 the first randomized trial of thymectomy in patients with non-thymomatous myasthenia gravis comparing extended transsternal thymectomy in conjunction with pharmacological prednisone therapy with prednisone therapy alone was published (1). A benefit regarding clinical outcomes in patients undergoing thymectomy was reported. So far there are no randomized trials delivering conclusive evidence on the value of minimally invasive techniques for thymectomy in patients with myasthenia gravis in comparison to the historic standard transsternal technique. The gap of missing high level evidence extends to patients with thymic epithelial tumors with and without myasthenia gravis despite of increasing numbers of patients undergoing surgery by means of different minimally invasive approaches with video-assisted thoracic surgery (VATS) and robotic assisted thoracoscopic surgery (RATS) (2). From a great number of different minimally invasive procedures for thymectomy can be chosen from. Decisions on the kind of equipment (VATS or RATS) and a preferred access site (left- or right-sided VATS/RATS, cervical or subxiphoid approach) vary greatly with institutional and the thoracic surgeons experience (3). Literature classifying the myriad of thymectomy techniques is available (4).
Before starting our RATS program a left sided three ports VATS combined with a small cervicotomy was our preferred approach in patients with myasthenia gravis and small tumors (5). At our institution the left sided robotic thymectomy technique became the standard approach for patients with myasthenia gravis and anterior mediastinal tumors (mainly thymic epithelial tumor) with sizes up to 4 cm starting in 2014 (6). We mainly favored the left sided approach to obtain better control of the left phrenic nerve and the innominate vein and its branches. Anterior mediastinal tumors with dimensions larger than 4 cm or radiological suspicion of tumor infiltration of organs neighboring the thymus were resected using mainly thoracotomy or sternotomy. In the presence of myasthenia gravis sternotomy was the standard technique.
To improve surgical radicality and reduce the length of surgical incisions surgeons proposed innovative additions to currently established minimally invasive techniques. Relevant for the current work was the addition of the subxiphoid approach as part of the procedure in order to improve “maximal” thymectomy (7); or putting the focus of surgical dissection at the subxiphoid approach, with just an extra right sided camera (VATS) support (8).
With increasing experience of extended thymectomy by robotic and video-assisted surgery on patients with myasthenia gravis we added modifications to the standard technique in patients with non-invasive thymic epithelial tumor sizes larger than 4 cm. While the minimally invasive resection in patients with tumors >4 cm was feasible, the specimen retrieval through the left intercostal camera port incision and right cardio-phrenic fatty tissue dissection (extended thymectomy in myasthenia gravis patients) remained technically challenging. The purpose of this prospective study was to evaluate the possibility of offering a modified minimally invasive approach as standard alternative for large non-invasive anterior mediastinal tumors in patients with or without myasthenia gravis.
Methods
Patient history and radiological examinations
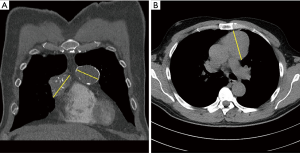
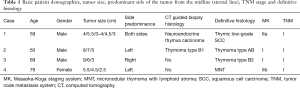
Three male and one female patient were referred to our thoracic surgery division at the Medical University Vienna. The patients had surgery between December 2016 and July 2017. In all cases anterior mediastinal tumor suspicion from routine chest X-ray examinations was confirmed by thoracic computed tomography scanning (Figure 1). Patients with non-invasive anterior mediastinal tumors with sizes greater than 4 cm were included in the study. All patients were asymptomatic and all patients were seen by a neurologist. None of the patients were diagnosed with myasthenia gravis. No previous cardiothoracic operations were carried out in any of the patients. In one case both thymic lobes were affected (5.5+4 cm lesions) and in the other three cases the tumor was predominantly on the left or right side of the sternal line (Table 1). In all cases the described left-sided VATS in conjunction with the subxiphoid approach was used (no right-sided VATS was used).
Full table
Technical specifications
V.A.T.S. instruments: ENDOEYE HD II Rigid Videoscope. Olympus.
Results
The technique
The surgical procedure can technically be divided into two phases (Figure 2):
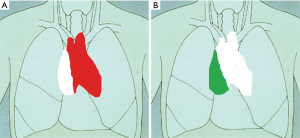
- Phase I. Peripheral venous access and continuous arterial monitoring was established by our anesthesiologists. A double lumen tube was positioned bronchoscopically. The patient was positioned in supine position on the left border of the operating table. Special attention was given to left arm position in order to decrease the height of the left shoulder. Two surgeons introduce the ports from the left side of the patient while the scrub nurse hands the instruments from the right patient side. During single lung ventilation of the right lung a 12 mm Trocar for insertion of the camera was placed in the 4th intercostal space at the level of middle axillary line; after correct placement was confirmed CO2 insufflation was started at levels ranging from 8–10 mmHg. Under direct camera vision two instrument ports were placed. An 8 mm port was placed at the 3rd intercostal space at the level of the anterior axillary line. Finally, the third port, also 8 mm in diameter, was placed at the 6th intercostal space at the level of the anterior clavicular line. The resection started dissecting the thymic tissue along the left phrenic nerve and pericardium until visualization of the innominate vein was achieved. Then the cervical ends of both thymic horns were carefully dissected and removed from the neck. All venous branches were ligated with 5 mm Endoclips. Finally, the right mammary vein was dissected from the surgical thymus (thymic gland plus mediastinal fatty tissue) and the right pleura was opened. In summary the first phase of extended thymectomy was performed under condition of CO2 insufflation to dissect the surgical thymus from the pericardium up to the middle line of the mediastinum.
- Phase II. The operation continues without CO2 support (also without sternal elevation or extra camera port from the right hemithorax). The surgeon moved to the right side of the patient (next to the scrub nurse), the second surgeon stayed on the left side. A 4 cm vertical incision with removal of the subxiphoid process was performed. A soft tissue retractor was inserted in the same way described for subxiphoid lung resections (9). During this phase the camera and two extra-long instruments were placed at the subxiphoid port; and one more traction instrument was inserted from the previous 6th intercostal space port. At this time, single lung ventilation was shifted from left to right. After collapse of the right lung, dissection started with the subxiphoid fatty tissue in order to visualize the right aspects of the mediastinum. All mediastinal attachments of the surgical thymus were divided under direct vision using a monopolar curved hook, to allow careful dissection of the pleura above the right phrenic nerve. After light lateral traction the whole surgical thymus was resected from the pericardium (“en bloc” resection of thymic gland, mediastinal tumor and mediastinal fatty tissue). The specimen retrieval was accomplished through the subxiphoid incision using a 15 mm endobag device (Medtronic Endocatch™ Gold Specimen Retrieval Pouch). The anterior mediastinal space and the right pleura ware drained through the left anterior port at the 6th intercostal space; and the subxiphoid incision layer by layer. Thereafter we closed the 3rd intercostal space port incision and inserted another drain at the level of the first phase camera port (4th intercostal space), placed as dorsal as possible for fluid drainage. With this drainage strategy we avoided bilateral intercostal nerve damage which might complicate the patient’s respiratory efforts in the early postoperative period (Figure 3).
Clinical characteristics
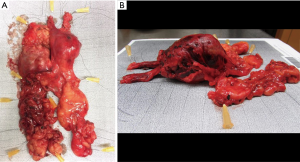
Operative time ranged from 117 to 151 minutes. Blood loss was less than 150 mL during all procedures. In two cases the final histopathological report revealed results differing from preoperative histology gained by computed tomography guided puncture (marked with * in Table 1). Patients were discharged on the third postoperative day with minimal discomfort at the subxiphoid region. All cases a complete resection with tumor free margins (R0) was achieved. The operative specimens of patients one and two are depicted in Figure 4. No major morbidity or mortality was observed during the 12 months follow-up period.
Discussion
Continuous shift from median/partial sternotomy (10) to more minimally invasive techniques was observed in the last two decades. The cervical approach became less popular as other well reproducible approaches achieved similar results avoiding the cosmetically disadvantageous neck incision (11,12). The potential use of subxiphoid approaches for thymic resections was based in publications in the 70s and 90s on subxiphoid mediastinal exploration for diagnostic purposes (13,14). In 1999, using a device to lift the sternum complete resections for benign tumors were reported (15). Further milestones with thoracic surgery from a lone standing subxiphoid incision or the combination of a subxiphoid incision with other procedures for patients with myasthenia gravis with or without small thymomas followed. In the recent past several sternal lifting devices were used to lift up the sternum at time of resection (7,16). With the continuous technical evolution, more sophisticated approaches, like RATS or microthymectomy were shown to yield acceptable results dealing with thymic resections (17). The use of single-port devices allowing CO2 insufflation paved the way for complete thymectomy using the subxiphoid approach in a “uni- or biportal way” (16,18). New surgical instruments with longer working length allowed uniportal extended thymectomy without use of CO2 insufflation (to avoid cardiac arrhythmias), but with more difficult control over the innominate vein and the left phrenic nerve (19).
The ongoing debate on which of the various minimally invasive techniques to adopt as an international standard is fueled by the development more and more modifications to innovations to current surgical practice. The described technique of sequential left sided VATS with completion of the procedure with the addition of a subxiphoid incision for right sided dissection for patients with non-invasive anterior mediastinal tumors larger than 4 cm TNM stage I (20) with or without myasthenia gravis was driven in an attempt to control the operative field at any part of VATS or RATS with maximal precision: improve the careful dissection of both phrenic nerves, gain optimal control over the small innominate vein branches draining the thymic gland. In cases of left sided dissection of tumors bigger than 4 cm, right sided dissection (especially control of the right phrenic nerve) and retrieval of the intact specimen for accurate pathological workup remained technically challenging. That was the reason we combined the left sides three ports VATS with the subxiphoid uniportal approach. The combination of the first phase left sided VATS for careful dissection of along the left phrenic nerve, the thymic horns up into the neck and the innominate vein branches with the second phase subxiphoid access for excellent right sided exposure to dissect along the right phrenic nerve, the right cardiophrenic fatty tissue and, at the end of the procedure the uncomplicated removal of the specimen through the subxiphoid incision. Another reason we prefer the subxiphoid access over a right sided camera port is the avoidance of possible bilateral intercostal nerve damage with associated pain hampering thoracic mechanics. The described VATS procedure can be adapted for RATS thymectomy of tumors larger than 4 cm in diameter.
Conclusions
We recommend the addition of a subxiphoid incision to left sided thymectomy VATS o RATS procedures attempting to remove non-invasive anterior mediastinal tumors with sizes above 4 cm. We believe that it is a valid tool to achieve excellent control over the right phrenic nerve and right cardiophrenic fatty tissue making extended thymectomy a more radial and safe procedure without adding significant comorbidity.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2018.09.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of the Medical University of Vienna confirms that such medical treatments that were carried out under the sole scope of decisions and responsibility of the treating physicians do not require the formal vote of its Ethics Committee. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med 2016;375:511-22. [Crossref] [PubMed]
- Ruffini E, Filosso PL, Guerrera F, et al. Optimal surgical approach to thymic malignancies: New trends challenging old dogmas. Lung Cancer 2018;118:161-70. [Crossref] [PubMed]
- Matilla JR, Klepetko W, Moser B. Thymic minimally invasive surgery: state of the art across the world-Europe. J Vis Surg 2017;3:70. [Crossref] [PubMed]
- Sonett JR, Jaretzki A 3rd. Thymectomy for nonthymomatous myasthenia gravis: a critical analysis. Ann N Y Acad Sci 2008;1132:315-28. [Crossref] [PubMed]
- Shigemura N, Shiono H, Inoue M, et al. Inclusion of the transcervical approach in video-assisted thoracoscopic extended thymectomy (VATET) for myasthenia gravis: a prospective trial. Surg Endosc 2006;20:1614-8. [Crossref] [PubMed]
- Mussi A, Fanucchi O, Davini F, et al. Robotic extended thymectomy for early-stage thymomas. Eur J Cardiothorac Surg 2012;41:e43-6; discussion e7.
- Zielinski M, Kuzdzal J, Szlubowski A, et al. Transcervical-subxiphoid-videothoracoscopic "maximal" thymectomy--operative technique and early results. Ann Thorac Surg 2004;78:404-9; discussion 9-10. [Crossref] [PubMed]
- Zielinski M, Czajkowski W, Gwozdz P, et al. Resection of thymomas with use of the new minimally-invasive technique of extended thymectomy performed through the subxiphoid-right video-thoracoscopic approach with double elevation of the sternum. Eur J Cardiothorac Surg 2013;44:e113-9; discussion e9.
- Song N, Zhao DP, Jiang L, et al. Subxiphoid uniportal video-assisted thoracoscopic surgery (VATS) for lobectomy: a report of 105 cases. J Thorac Dis 2016;8:S251-7. [PubMed]
- Ruffini E, Guerrera F, Filosso PL, et al. Extended transcervical thymectomy with partial upper sternotomy: results in non-thymomatous patients with myasthenia gravis. Eur J Cardiothorac Surg 2015;48:448-54. [Crossref] [PubMed]
- Bril V, Kojic J, Ilse WK, et al. Long-term clinical outcome after transcervical thymectomy for myasthenia gravis. Ann Thorac Surg 1998;65:1520-2. [Crossref] [PubMed]
- Slater G, Papatestas AE, Kornfeld P, et al. Transcervical thymectomy for thymoma in myasthenia gravis. Am J Surg 1982;144:254-6. [Crossref] [PubMed]
- Arom KV, Franz JL, Grover FL, et al. Subxiphoid anterior mediastinal exploration. Ann Thorac Surg 1977;24:289-90. [Crossref] [PubMed]
- Hutter J, Junger W, Miller K, et al. Subxiphoidal videomediastinoscopy for diagnostic access to the anterior mediastinum. Ann Thorac Surg 1998;66:1427-8. [Crossref] [PubMed]
- Kido T, Hazama K, Inoue Y, et al. Resection of anterior mediastinal masses through an infrasternal approach. Ann Thorac Surg 1999;67:263-5. [Crossref] [PubMed]
- Suda T, Sugimura H, Tochii D, et al. Single-port thymectomy through an infrasternal approach. Ann Thorac Surg 2012;93:334-6. [Crossref] [PubMed]
- Dunning J. Video-assisted thoracoscopic microthymectomy. Ann Cardiothorac Surg 2015;4:550-5. [PubMed]
- Suda T, Ashikari S, Tochii D, et al. Dual-port thymectomy using subxiphoid approach. Gen Thorac Cardiovasc Surg 2014;62:570-2. [Crossref] [PubMed]
- Wu L, Lin L, Liu M, et al. Subxiphoid uniportal thoracoscopic extended thymectomy. J Thorac Dis 2015;7:1658-60. [PubMed]
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S65-72. [Crossref] [PubMed]
Cite this article as: Matilla JR, Alvoeiro M, Benazzo A, Schwarz S, Klepetko W, Moser B. Initial experience with a combined sequential left-sided and subxiphoid video-assisted thoracic surgery approach for resection of large anterior mediastinal tumors. Mediastinum 2018;2:58.