
Expression patterns and prognostic value of programmed death ligand-1 and programmed death 1 in thymoma and thymic carcinoma
Thymic epithelial tumors (TETs) arising in the anterior mediastinum are rare malignancies that account for less than 1% of all adult cancers (1). The incidence of TETs is 1.5 to 3.2 per 1,000,000 person-years (2,3). The International Thymic Malignancy Interest Group database including more than 6,000 patients showed that thymoma and thymic carcinoma accounted for 81% and 14%, respectively, of all TETs (4). Of these, 63%, 23%, and 13% of patients had local (stage I–II), locally advanced (stage III), and disseminated or distant metastatic (stage IV) disease, respectively (4). These figures are consistent with those from population-based studies (2,5).
Complete resection is the curative treatment that assures long-term survival in patients with resectable disease, whereas for patients with disseminated disease or distant metastases systemic chemotherapy is the mainstay of treatment. A combination of carboplatin plus paclitaxel has been used widely, but the response rate and median progression-free survival (PFS) are 43% and 16.7 months, respectively, in patients with advanced thymoma and 22% to 36% and 5 to 7.5 months, respectively, in patients with thymic carcinoma (6,7).
The limitation of conventional chemotherapy has prompted medical oncologists to develop molecular targeted therapies in patients with advanced TET. Recently, early results of phase II trials of antibody therapies that target programmed death 1 (PD-1) were reported. Phase II trials of pembrolizumab in patients with recurrent thymic carcinoma who had progressed after at least one line of chemotherapy showed an objective response in about 20% of patients and stable disease in more than half of patients with a median PFS of 4 to 6 months (8,9). In contrast, a phase II trial of nivolumab in patients with unresectable or recurrent thymic carcinoma with progression after at least one previous platinum-based chemotherapy found no objective response in 15 patients for the first stage with a median PFS of 3.8 [95% confidence interval (CI), 1.9–5.6] months, resulting in early termination of accrual to this study (10). This discrepancy among the trials, as well as among patients, can be explained by the heterogeneity of thymic carcinoma among patients, which should be assessed by adequate biomarkers. Programmed death ligand-1 (PD-L1) expression on tumor cells and PD-1 expression on tumor-infiltrating lymphocytes (TILs) are candidates for such a biomarker, because an association between these markers and survival has been observed in some tumor types (11).
Owen et al. investigated the expression patterns of PD-L1 and PD-1 and their association with clinical and pathological parameters in thymoma and thymic carcinoma (12). They assessed PD-L1 and PD-1 expression by immunohistochemistry using anti-PD-L1 antibody (22C3) and anti-PD-1 antibody (NAT105) and graded the expression in a semi quantitative 0–5 scoring system (0= no expression; 1= rare; 2= low; 3= moderate; 4= high; and 5= very high). PD-L1 expression (score ≥1) was detected in 81% (26/32) of thymoma and 100% (3/3) of thymic carcinoma samples. PD-1 expression was noted in 81% of thymoma and 33% of thymic carcinoma samples. Multiple slides prepared from the same tumor specimen in three patients with thymoma demonstrated intra-tumoral heterogeneity in terms of PD-L1 and PD-1 expression. Inconsistent with results from previous papers, neither the PD-L1 expression nor the PD-1 expression was associated with the pathological WHO grade, tumor stage, or overall survival.
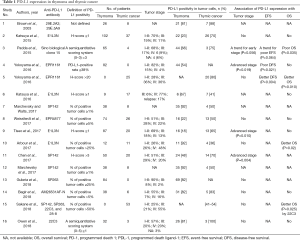
PD-L1 expression in tumor cells varies in the literature with studies showing expression from 23% to 92% in thymoma and 36% to 100% in thymic carcinoma (Table 1). When comparing the PD-L1 expression between thymoma and thymic carcinoma within a single study, some studies showed that PD-L1 expression was higher in thymoma than in thymic carcinoma, but others showed that PD-L1 expression was higher in thymic carcinoma than in thymoma. Its association with tumor stage was also inconsistent between studies; PD-L1 expression was associated with advanced stage or early stage in some studies, but most studies showed no association with tumor stage.
Full table
How can we explain these discrepant results? They may be attributable to the different PD-L1 immunohistochemical assays used in each study (Table 1). In a comparison of four PD-L1 assays with different antibodies (SP142, SP263, 22C3, and 28-8), as high as 47% of cases showed discordance between PD-L1 expression levels (13). The assessment method of PD-L1 expression and definition of PD-L1 positivity also varies among studies. A semiquantitative scoring system was used in two studies, a percentage of positive tumor cells in six studies, a percentage of the PD-L1—positive area/cytokeratin-positive area in one study, and H-score in five studies. Considering the intratumoral heterogeneity of PD-L1 expression, H-score may be better than the other methods, but it was used in less than one-third of studies. The cutoff value of PD-L1 positivity is also not uniform. The threshold for PD-L1 positivity ranged from 1% to 50% when examining the percentage of positive tumor cells and from 1 to 20 when using the H-score.
Interpretation of PD-1 expression in TILs is more complicated, because identification of TILs is difficult. No studies used multiplex immunohistochemistry with antibodies for T-lymphocyte identification. In addition, methods for evaluating the PD-1 expression also vary between studies, as with PD-L1 expression (Table 2).

Full table
The association of PD-L1 and PD-1 expression with survival is also conflicting (Tables 1,2). It is difficult to assess the prognostic value of PD-L1 and PD-1 expression in these studies, because established prognostic factors, including the pathological WHO grade and tumor staging system of TETs, were not controlled. Small sample size was also an issue.
In conclusion, establishing biomarkers to select eligible patients for PD-1/PD-L1 pathway inhibitors is an immediate need in the treatment of advanced TETs. PD-L1 expression on tumor cells and PD-1 expression on TILs are clearly candidates for this purpose, but recently obtained clinical data are not sufficient to support their use as biomarkers. A PD-1/PD-L1 immunohistochemical assay with an optimal antibody, an assessment method of staining, and definition of positivity should be standardized in future clinical studies.
Acknowledgments
We would like to thank Editage (
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by Section Editor Dr. Zhuoqi Jia (Thoracic Department, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2018.08.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Travis WD, Brambilla E, Burke AP, et al. Pathology and Genetics: Tumours of the Lung, Pleura, Thymus and Heart. France, Lyon: IARC, 2004.
- de Jong WK, Blaauwgeers JL, Schaapveld M, et al. Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur J Cancer 2008;44:123-30. [Crossref] [PubMed]
- Siesling S, van der Zwan JM, Izarzugaza I, et al. Rare thoracic cancers, including peritoneum mesothelioma. Eur J Cancer 2012;48:949-60. [Crossref] [PubMed]
- Huang J, Ahmad U, Antonicelli A, et al. Development of the international thymic malignancy interest group international database: an unprecedented resource for the study of a rare group of tumors. J Thorac Oncol 2014;9:1573-8. [Crossref] [PubMed]
- Mariano C, Ionescu DN, Cheung WY, et al. Thymoma: a population-based study of the management and outcomes for the province of British Columbia. J Thorac Oncol 2013;8:109-17. [Crossref] [PubMed]
- Lemma GL, Lee JW, Aisner SC, et al. Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma. J Clin Oncol 2011;29:2060-5. [Crossref] [PubMed]
- Hirai F, Yamanaka T, Taguchi K, et al. A multicenter phase II study of carboplatin and paclitaxel for advanced thymic carcinoma: WJOG4207L. Ann Oncol 2015;26:363-8. [Crossref] [PubMed]
- Giaccone G, Kim C, Thompson J, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol 2018;19:347-55. [Crossref] [PubMed]
- Cho J, Kim HS, Ku BM, et al. Pembrolizumab for Patients With Refractory or Relapsed Thymic Epithelial Tumor: An Open-Label Phase II Trial. J Clin Oncol 2018;JCO2017773184 [PubMed]
- Seto T, Katsuya Y, Horinouchi H, et al. Primary result of an investigator-initiated phase II trial of nivolumab for unresectable or recurrent thymic carcinoma: PRIMER study (NCCH1505). J Thorac Oncol 2018;13:S61-2. [Crossref]
- Zhang Y, Kang S, Shen J, et al. Prognostic significance of programmed cell death 1 (PD-1) or PD-1 ligand 1 (PD-L1) Expression in epithelial-originated cancer: a meta-analysis. Medicine (Baltimore) 2015;94:e515 [Crossref] [PubMed]
- Owen D, Chu B, Lehman AM, et al. Expression Patterns, Prognostic Value, and Intratumoral Heterogeneity of PD-L1 and PD-1 in Thymoma and Thymic Carcinoma. J Thorac Oncol 2018;13:1204-12. [Crossref] [PubMed]
- Sakane T, Murase T, Okuda K, et al. A comparative study of PD-L1 immunohistochemical assays with four reliable antibodies in thymic carcinoma. Oncotarget 2018;9:6993-7009. [Crossref] [PubMed]
Cite this article as: Sekine I, Aida Y, Suzuki H. Expression patterns and prognostic value of programmed death ligand-1 and programmed death 1 in thymoma and thymic carcinoma. Mediastinum 2018;2:54.


