
Case presentations and recommendations from the October 2017 ITMIG tumor board: an international multidisciplinary team
Introduction
The International Thymic Malignancy Interest Group (ITMIG) is an academic society that has periodic virtual tumor board meetings to discuss challenging cases. The tumor board is composed of a moderator and at least one of the following clinicians; thoracic surgeon, medical oncologist, radiation oncologist (RO), diagnostic radiologist, and thoracic pathologist. Challenging cases and clinical questions are submitted by treating clinicians to get an expert opinion from multiple specialists, all with significant interest and experience in thymic malignancies. Following the case discussion, the tumor board summarizes its conclusions in writing for the treating clinician to help guide them in their treatment plan. We present two cases discussed at our virtual tumor board meeting that was conducted in October 2017. This group attempts to meet once a month with representation from multiple specialties, on this occasion the following were present: surgical oncology, medical oncology, radiation oncology, pathology, and radiology.
Case presentation 1
A 72-year-old woman presented to the Emergency Department after a fall complaining of bilateral leg weakness. She had polio as a child, but no other contributing history. On physical examination her vital signs were normal as well as her neurological examination. She had normal reflexes, no neurological deficits, and no leg weakness.
As part of the routine investigation, a chest radiograph was obtained which demonstrated a prevascular mass abutting and inseparable from the right heart border (Figure 1A). To further investigate this mass, a contrast-enhanced chest CT scan (Figure 1B) was obtained followed by a chest MRI (Figure 1C,D). Upon review of the images the tumor board radiologist described the mass as a homogenous fluid density prevascular mass, most suggestive of a benign cyst. Although radiologists and clinicians are more comfortable interpreting chest CT scans, MRI is superior to chest CT when investigating cystic lesions (1). This is due to MRI’s superior contrast resolution, which enables better differentiation between soft tissue tumors and fluid, and enables one to detect soft tissue within cysts. When dealing with prevascular mediastinal masses this is important, as benign cysts typically have imperceptible walls and no nodularity within them, whereas cystic thymomas, which may at first seem to mimic a simple cyst, actually have soft tissue nodularity and/or a thickened septum or wall, which enhance after contrast injection (2). The MRI was reviewed and showed that indeed the tumor was of fluid density, low on T1-weighted images, and high on T2-weighted images with no thickened septa and no nodularity (Figure 1C,D). Thus, the prevascular mass had the typical imaging features of a benign cyst, which may have arisen from the thymic bed as a thymic cyst, or the pericardium as a pericardial cyst.
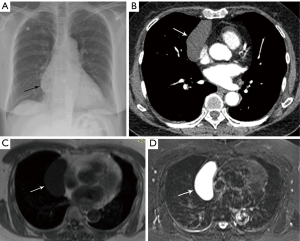
The initial reviewing radiologists had come to the same conclusion that this was a simple thymic cyst and therefore a biopsy was not recommended, and the patient was told the diagnosis by her RO. She was reassured that no intervention was required for this benign condition.
Questions for ITMIG tumor board
Three months after the diagnosis the patient’s daughter called the attending RO to report the patient had been having intermittent chest pain. She had never had chest pain previously and the patient was very concerned that this was related to the mass. The RO presented her case to the ITMIG group with the following questions: (I) would anyone in the group suggest surgical resection, and (II) if not how frequently should the mass be imaged and with what modality for observation?
ITMIG expert opinions
In the groups expert opinion there was adequate imaging that was consistent with a simple thymic cyst. Therefore, the mass is not contributing to her symptoms and there may be a component of psychological overlay if other causes of chest pain are unlikely or ruled out. In this case she should be reassured that surgery is not required. Additionally, the group does not recommend ongoing observation with imaging. If the managing RO believes further imaging is necessary for patient reassurance an annual MRI would be the frequency and modality of choice.
Discussion
If a man or woman over the age of 40 presents with a prevascular mediastinal mass that on CT imaging is well-circumscribed, round/oval/saccular, and homogeneous located near the thymic area, this is a highly characteristic lesion for a thymic cyst and requires evaluation with an MRI (3). If purely cystic this is determined to be a unilocular thymic cyst with a very high level of confidence that can be diagnosed with imaging alone and does not require tissue diagnosis (3). It is thought to be a relatively rare diagnosis which makes up <5% of prevascular mediastinal masses, though this is based on surgical series and not on the prevalence of incidentally detected cysts which are often not resected. In fact, benign thymic cysts are often mistaken for thymomas and excised when MRI is not used for their investigation, as sometimes CT may not be able to distinguish them from solid masses (4). Such cysts do not require resection, as they are benign. Common practice is to follow these cysts with MRI for about 2 years (3).
Case presentation 2
As part of his routine medical check-up, a 51-year-old asymptomatic marathon runner had a chest radiograph, which revealed a mediastinal mass. His physical examination was normal. Four years before that he had had pericarditis which was diagnosed by clinical symptomatology and echocardiography, presumed to be of viral etiology, and the patient recovered with non-steroidal anti-inflammatory medication.
Following the chest radiograph, the patient had a chest CT, chest MRI, and whole body FDG PET-CT that were reviewed by the diagnostic radiologist on the tumor board. The chest CT demonstrates a prevascular soft tissue mass (Figure 2A) which abuts the right atrium. Taking into account the patient being asymptomatic and his age, this solitary prevascular smooth soft tissue mass most likely represents a thymoma. Since no definite fat separates the tumor from the right atrium, it is difficult to know if the tumor simply abuts and does not involve the pericardium and right atrium, or if the tumor involves them. The MRI demonstrates that at certain levels (Figure 2B) there is a fat plane separating the tumor from the right atrium, and thus no right atrial involvement, but at other levels (Figure 2C), there is no such separation and the tumor even indents the right atrium. It is impossible from these images alone to know if the right atrium is involved or only abuts but is separable from the tumor. MRI cine images, in motion (not shown), showed that the mass does not move with the cardiac contractions, demonstrating that it is not attached to the right atrium. As for the pericardium, it is impossible to say if it only abuts the tumor or is actually involved by it, which will have to be determined by a pathologist. The tumor is FDG avid (Figure 2D) and no distant FDG avid metastases were identified. The use of FDG PET-CT is not routine with thymomas as not all are FDG avid. When the FDG uptake is very high, this often goes with more aggressive thymic tumors such as thymic carcinoma and type B3 disease.
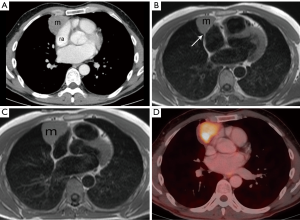
The patient had unremarkable complete blood count (CBC), coagulation, urine test, echocardiogram, and spirometry. The echocardiograph (ECG) demonstrated complete right bundle branch block that was not present 2 years prior. Tumor markers were done to rule out a germ cell tumor and alpha-fetoprotein (AFP), beta human chorionic gonadotropin (hCG), carcinoembryonic antigen (CEA), and lactate dehydrogenase (LDH) were all within the normal range.
The patient was diagnosed with a thymoma and underwent surgical resection 3 weeks after the initial finding. The surgery protocol was for complete resection of the thymoma (R0 resection). The presenting ITMIG member was the surgeon and he reported the mass was resected easily, was not attached to the pleura, and capsule of the tumor was completely preserved with the specimen.
The available images (Figure 3) show a circumscribed neoplasm that focally invades into the surrounding adipose tissue (Figure 3A,B). The neoplasm is comprised of a mixture of small lymphocytes and large epithelial cells (Figure 3C,D), some of which are clustering (Figure 3E,F). The morphologic features are consistent with a minimally invasive WHO type B2 thymoma. This is supported by multiple keratin immunostains, including pancytokeratin and high molecular weight cytokeratin that highlight a meshwork of epithelial tumor cells (Figure 3H,I). CD5 marks T cells (Figure 3J). A large fragment of thymoma is identified within a vessel outside of the tumor (Figure 3G). However, this fragment shows no definite attachment to the vessel wall and therefore it might not present true angiolymphatic invasion. The pathologic tumor stage is pT1a, stage I (TNM staging) or Masaoka-Koga stage IIa.
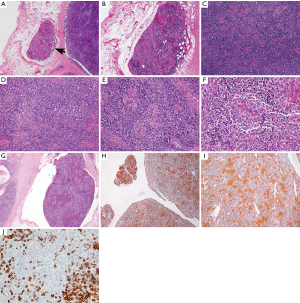
Questions for ITMIG tumor board
The questions for the tumor board were: (I) is the final diagnosis Thymoma B2, Masaoka stage IIa? (II) the patient was advised he did not require post-operative radiotherapy but would undergo close surveillance, would the group advise differently? (III) what are the chances of recurrence? (IV) what is the implication of the “microscopic foci of lymphatic vascular invasion” described in the pathology report? and finally (V) what are the follow up recommendations?
ITMIG expert opinions
The group agrees with the stage IIa tumor diagnosis as per the Masaoka-Koga staging system since pathology demonstrated microscopic transcapsular invasion (5). The tumor is type B2 (intermediate increasing epithelial/lymphocyte ratio and emergence of atypia) by the World Health Organization Histopathological Classification of Thymomas (6). With regards to indication for post-operative radiotherapy the ITMIG RO indicated there are no clear guidelines, however with an R0 resection and no concerns since the margin is 5 mm there would likely be no benefit of adjuvant radiotherapy for the patient, however if there was invasion of other structures upstaging the tumor to stage III then this is less clear. As noted by the ITMIG pathologist, a large fragment of thymoma is identified within a vessel outside of the tumor, however it demonstrates no definite attachment to the vessel wall and therefore it might not present true angiolymphatic invasion. Therefore, the group recommended no additional treatment since there is no concern that the patient is at increased risk of recurrence since there is a clear margin and confirmed stage IIa disease, however, the patient should be advised that adjuvant radiotherapy could reduce the local recurrence rate but would not impact his overall survival. As per the ESMO surveillance protocol published in 2015 the patient will require serial imaging, specifically the group proposed thoracic imaging annually for 5 years, then decrease the frequency to every 2 years for 5 years (7). In regards to whether to follow the patient with serial CT scans or MRI scans, the sternotomy wires may produce some artifacts in the retrosternal region but these are quite limited to that area. It is thus recommended that the patient has a baseline postoperative chest CT scan, with later follow-ups, which could be performed with either CT or MRI to decrease cumulative radiation dose.
Discussion
Many patients with Masaoka-Koga stage I or II tumors die of causes other than thymoma, and patients can also live for many years despite a recurrence due to the indolent behaviour of this disease (8). Patients with stage IIa disease who have a complete resection have a 10-year overall survival of 75–85%, and type B2 patients have a 10-year disease-free survival of 85% (9). The mean time to recurrence of a completely resected thymoma, which is a major prognostic factor, is approximately 5 years, however for stages II–IV it is 3 years (8). After tumor stage, complete resection is the most significant prognostic factor for progression free and overall survival (9). Postoperative radiotherapy is recommended in incompletely resected thymomas, and completely resected stage II and III thymomas may also benefit from adjuvant radiotherapy to reduce local recurrence rates but there has been no impact on survival demonstrated (9). At a minimum, follow-up imaging should include CT scans of the thorax annually for 5 years after surgical resection, then alternating annually with a chest radiograph until year 11, and then annual chest radiographs alone (8). Obtaining a new baseline examination 4–12 weeks postoperatively is recommended (8). MRI may be useful instead of CT either for better visualization or to minimize exposure to radiation, especially in younger patients (8).
Acknowledgments
We would like to thank all of the ITMIG members who helped start this collaborative initiative and the colleagues present for the meeting who made the discussion and this publication possible, including P. Bruce and Dr. J Clavero Ribes.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ med.2018.07.03). ACR serves as an unpaid Associate Editor of Mediastinum from May 2017 to Apr 2019. CBF serves as an unpaid editorial board member of Mediastinum from May 2017 to Apr 2019. EMM reports honorarium for lecture from Bristoll-Meyers Squibb, Boehringer Ingelheim, and Merck Sharp and Dohme, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tomiyama N, Honda O, Tsubamoto M, et al. Anterior mediastinal tumors: diagnostic accuracy of CT and MRI. Eur J Radiol 2009;69:280-8. [Crossref] [PubMed]
- Ackman JB, Wu CC. MRI of the thymus. AJR Am J Roentgenol 2011;197:W15-20 [Crossref] [PubMed]
- Carter BW, Marom EM, Detterbeck FC. Approaching the patient with an anterior mediastinal mass: a guide for clinicians. J Thorac Oncol 2014;9:S102-9. [Crossref] [PubMed]
- Ackman JB, Verzosa S, Kovach AE, et al. High rate of unnecessary thymectomy and its cause. Can computed tomography distinguish thymoma, lymphoma, thymic hyperplasia, and thymic cysts?. Eur J Radiol 2015;84:524-33. [Crossref] [PubMed]
- Koga K, Matsuno Y, Noguchi M, et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int 1994;44:359-67. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Girard N, Ruffini E, Marx A, et al. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v40-55. [Crossref] [PubMed]
- Huang J, Detterbeck FC, Wang Z, et al. Standard outcome measures for thymic malignancies. J Thorac Oncol 2011;6:S1691-7. [Crossref] [PubMed]
- Girard N, Mornex F, Van Houtte P, et al. Thymoma: a focus on current therapeutic management. J Thorac Oncol 2009;4:119-26. [Crossref] [PubMed]
Cite this article as: Sigurdson S, Marom EM, Roden AC, Detterbeck FC, Falkson CB. Case presentations and recommendations from the October 2017 ITMIG tumor board: an international multidisciplinary team. Mediastinum 2018;2:53.


