
Thymoma and thymic carcinoma: a perspective on the NCCN clinical practice guidelines in oncology
Introduction
Although there seems to be great attention in recent years about the occurrence, diagnosis, staging, and nomenclature of thymic tumors, namely thymomas and to some extent thymic carcinoma, the reality is that thymic tumors represent a poorly understood group of tumors that over the years have been not only misdiagnosed for other conditions—granulomatous thymoma for Hodgkin lymphoma or seminomatous thymoma for Seminoma, but also more recently engaged in a rather unscientific nomenclature such as the use of letters and numbers, which only evidence a deepening in the lack of understanding for these tumors. If to the issue of nomenclature, we add the issue of staging, which incorrectly the same schema has been used for two different tumoral conditions. Then, we can easily observe that the field of thymic tumors—thymoma and thymic carcinoma, is still poorly understood. There are compelling reasons why the state of thymoma and thymic carcinoma has remained obscure: (I) the occurrence of these tumors is rare, (II) the legacy of understanding these tumors has been poor at best, (III) the incorrect approach to unify thymoma and thymic carcinoma as a single entity, (IV) the proposal of nomenclatures that have more political undertones than scientific approach, (V) the proposal of staging systems that appear to represent “virtual proposals”, based on theory rather than facts, and (VI) the lack of a “true” panel of experts who can shed some meaningful scientific light into this area without personal agendas.
Regarding the NCCN guidelines, it seems that they are primarily for treating physicians rather than pathologists, however, if this premise is correct, then why include criteria for the diagnosis and/or nomenclature on the histological classification of those tumors. These guidelines in a snapshot are the reflection of the current disconnect between pathology and medical oncology when it comes to the understanding of thymoma and thymic carcinoma. These guidelines strictly from the pathology diagnosis of thymoma not only are framed in an award way but also, they may be more appropriate for medical students than for experienced surgical pathologists, needless to say for the experienced thoracic pathologist. The inclusion of obligatory and optional criteria does not make sense, for instance would an oncologist or surgeon not accept the diagnosis of thymoma simply because the pathologist did not perform an immunohistochemical stain for Tdt to demonstrate immature lymphocytes? One can enumerate more issues with these obligatory and optional criteria that appear to have been written by individuals with no experience on thymoma.
Nomenclature and staging
Prior to the histological classification proposed by Bernatz in 1961 (1), the nomenclature of thymomas was essentially incorrect all the way around. Bernatz et al. (1,2) proposed a simple but workable classification of thymomas based on the proportion of lymphocytes and to some extent based on the shaped of the epithelial cells, thus, epithelial rich and lymphocyte rich thymomas or spindle cell thymomas. Important points to highlight from this classification system:
- it is based on surgical resections;
- more than 100 cases;
- more importantly, this histological classification does not attempt to predict prognosis based on histology but stresses the issue of tumor invasion as the most important factor of clinical outcome.
This approach is essentially an echo of other reports stressing the heterogeneity of thymomas (3), a fact that conveniently is still being ignored by many. The Bernatz classification system (1,2), even today has endured the test of reproducibility in the majority of cases.
In 1985, Marino and Muller-Hermelink (4) reported a study of 58 thymomas and 13 thymic carcinomas and proposed the so-called histogenetic classification: cortical, medullary, and mixed thymoma. Important issues to highlight from this proposal:
- the proposal was made on biopsy specimens;
- it is argued that the cell of origin of medullary thymoma comes from the medulla of the thymus and for cortical thymoma from the epithelial cells of the cortex of the thymus;
- this proposal states that histology is predictive of prognosis;
- only three biopsy samples belong to the medullary thymoma (spindle cell thymoma) and based on those three samples the grand statement that those tumors are benign came to live.
Needless to say, such proposal is unprecedented in modern surgical pathology. Most of this theoretical schema has not been proven even by the proponents of the schema (5,6), and some of the assumptions have also been proven incorrect (7,8).
By the time the World Health Organization (WHO) became part of this struggle, the cards have already been dealt with and the members of that panel in 1999 (9) considered essentially only two schemas: the Bernatz from 1961 and the Marino-Muller-Hermelink of 1985. As a consequence, a “compromise” the letters and numbers to create a “translator” for these two approaches. It was stated in the 1999 (9) that the letters and numbers do not represent a classifications system, a detail that has been conveniently ignored by the authors of two subsequent WHO publications in 2004 and 2015.
Contrary to the Bernatz, Marino-Muller-Hermelink, and WHO, we have maintained that the histological variants of WHO type A, B1, and B2 have essentially similar clinical behavior, which renders this schema impractical, a point that has also been proven by others in larger series of cases. What we consider is the most important aspect is to separate “Atypical Thymoma” (WHO B3) as this tumor even though is less frequent than the other histological variants, has a tendency to be more aggressive as the majority of tumors are invasive at the time of diagnosis (10-14). Therefore, the concept of thymoma—atypical thymoma—thymic carcinoma.
Regarding staging of thymomas, there are currently two schemas that even though have a descriptive approach in terms of the anatomical distribution of the tumor, both of these schemas offered meaningful clinical results (15-17). However, there are important differences in it. The Masaoka staging system modified by Koga—stages I and II have similar survival rates as has been observed in meta-analysis comparing those stages (18). On the contrary, the Moran staging system highlights the importance of limited disease (stages 0 and I) contrary to invasive disease (stages II and III). More importantly, it highlights the issue that stage 0 represents the equivalent to an in-situ neoplasm. This staging system has been recently validated with 1,470 thymoma resections in which the system was used (14).
Regarding thymic carcinoma, the pathology community is not confused as we clearly separate thymoma from thymic carcinoma and its different variants. However, unfortunately in previous schemas presented for thymomas the use of names such as “malignant thymoma” has been equated with thymic carcinoma, which even today, it is unfortunately used by many and something that continues creating a problem in the proper nomenclature of these tumors. At this point, it is highly important to make an unequivocal statement: malignant thymoma whatever that concept may be does not mean thymic carcinoma. It is very likely that the concept of “malignant thymoma” was meant to be used as equivalent to “invasive thymoma.” In addition, it is important to highlight that the majority of thymic carcinomas are of the squamous type and truly represent a clinico-radiological correlation. Pathologically speaking, there is nothing pathognomonic about thymic carcinoma and its diagnosis requires strict radiological evidence of a mediastinal mass (19,20). Contrary to the use of the Masaoka staging system proposed for thymomas, thymic carcinomas on the other hand commonly involve lymph nodes. Therefore, it is inappropriate to use the Masaoka staging system. Actually for thymic carcinoma, a TNM system is more helpful and one that has also been presented in the literature (21), and conveniently ignored by those who still today with abundant literature on the topic of thymoma and thymic carcinoma see these two neoplasms as the same. It has been demonstrated that in thymic carcinoma, the presence of lymph node metastasis alters the survival rate in these patients (21).
Based on that background, now the NCCN stablishes guidelines for thymoma and thymic carcinoma (22). Let us examine in closer detail their position in these subjects of thymoma and thymic carcinoma:
- The authors grouped together under RO resection thymoma, no capsular invasion or thymic carcinoma, stage I (likely using Masaoka’ staging). These two tumors even though may be encapsulated very likely do not follow the same clinical behavior. While an encapsulated thymoma likely will be cured by complete surgical resection, the same cannot be stated for thymic carcinoma. The issue of capsular integrity is valid for thymoma but not necessarily for thymic carcinoma that often is an irregular mass rather than the well-defined tumor mass often observed in thymoma. However, this grouping clearly puts in evidence the erroneous concept of considering thymoma and thymic carcinoma as equals. In addition, it is not clear to what type of thymic carcinoma the authors are referring? Adenocarcinoma? Neuroendocrine carcinoma? Anaplastic carcinoma? Spindle cell carcinoma? Squamous carcinoma?
- Also, as R0 the authors place thymoma or thymic carcinoma, capsular invasion present stages II-IV. Here, the issue is even more concerning. A minimally invasive thymoma is well known to behave like an encapsulated thymoma (14). However, the same degree of invasion in thymic carcinoma likely has a different clinical outcome. Needless to say, when thymic carcinoma or thymoma is in late stages, the clinical outcome is different despite complete surgical resection;
- The authors get in the diagnostic features of thymoma based on what the authors consider the WHO guidelines, which over and over have been found at best impractical. But more interestingly, the NCCN authors just like the authors of the WHO, have mixed one classification system with the WHO translator. Atypical thymoma is part of the concept developed by Suster-Moran in their classification for thymoma, atypical thymoma, it is not a concept of the WHO, which uses letters and numbers, and tacitly has endorsed the concept of predicting prognosis by histology, so that the concept of Atypical A, although understood, makes no sense whatsoever, unless you use the Suster-Moran classification system. In addition, the authors include obligatory criteria and optional criteria. In the obligatory criteria, the author includes the use of immunohistochemical stains, which for an experience pathologist with thymomas, the use of such aid is completely not needed. Furthermore, in the optional criteria the authors include the presence of Hassall’s corpuscles and perivascular spaces. This is exactly the way that pathologists do not practice pathology. We look at the overall pattern and detail of the tumor in question and based on whatever is present, then a diagnosis is made. These guidelines probably are meant to non-pathologists or medical students because if they are for practicing pathologists, then they are somewhat ambiguous at best and if they are meant for experience pathologists in mediastinal pathology these guidelines are just flat wrong;
- The authors enter micronodular thymoma (MNT). These tumors for the experience pathologist in mediastinal tumors represent a growth pattern easily recognized that just happens to have B-cell lymphoid hyperplasia (23). The importance of recognizing such entity is that in the past it was misdiagnosed as metastatic thymoma to lymph node. In addition, there is a variant of this pattern that corresponds to Micronodular thymic carcinoma (24). Once again, the morphology of the tumor is distinct enough to base the diagnosis on morphological grounds;
- The authors include another variant of thymoma under metaplastic thymoma, a renamed based on nothing more than the sake of changing the name of tumors. The original description of such tumor is clear “thymomas with pseudosarcomatous stroma.” (25). Later on, incorrectly name as metaplastic carcinoma (26). Perhaps the authors of the NCCN the same as those of the WHO consider that even though one concept is wrong (metaplastic carcinoma) the use of the sexy name of metaplastic makes the entity correct;
- Rare others—in our experience those “rare others” represent a meaningful percentage of cases that commonly are misdiagnosed for other conditions. Here is where the authors of these NCCN guidelines should have come up with obligatory criteria. As here is where a correct diagnosis has an impact of patients’ prognosis. In this component, the authors of the NCCN completely missed the most important aspect of the diagnosis of thymoma, but then again, perhaps the authors of these NCCN guidelines do not have the needed experience in the diagnosis of unusual thymomas;
- The authors quoted the Masaoka staging system. However, what is quoted here is not the original Masaoka or the original Koga. Perhaps here the authors intended to paraphrased or idealized the Masaoka staging system. The original Masaoka staging is in Roman numerals and Arabic numerals, thus stage I is subdivided into 1 and 2. However, the Koga modification of Masaoka was only in one component and that is for stage II-2 Microscopic invasion into capsule for invasion thorough the capsule. However, here is a bigger problem that the NCCN authors did not address and that was addressed in the Moran staging system (14,17). The problem is one of possible semantics: in Masaoka stage II-1—macroscopic invasion into surrounding fatty tissue or mediastinal pleura—compared to stage IVa—pleural or pericardial dissemination. It is well known that at the initial diagnosis of thymomas, the tumor may invade the pleura but do not disseminate the pleura. Pleural or pericardial dissemination is more commonly seen in recurrences of thymoma but not in the initial diagnosis. However, in the Masaoka schema a patient can be easily moved from stage II-1 to stage IVa, just for the application of one word—invasion or dissemination. Once again, the authors of the NCCN guidelines had the opportunity to make corrections and simply kept the status quo. Perhaps because now they are endorsing a TNM staging, which interestingly borrows similar definitions as the Masaoka and Moran schemas;
- The authors entered the newly created TNM schema based on ideals rather than hard data as there is not a single series of cases using such TNM system. However, one can argue that since this TNM proposal truly borrows from the Masaoka and Moran schemas their definitions, then there may not need to be a series of cases. On the other hand, one can argue that if that is not needed, then perhaps a TNM is the one that is not needed as the other schemas are functioning well for the most part. What we know and also acknowledged by the authors of the NCCN guidelines is that thymomas rarely if at all metastasize to lymph nodes. In our experience, the great majority of cases, a tumor nodule from an invasive thymoma is obtained and mistaken for a possible lymph node but it does not represent a lymph node, just an invasive tumor nodule. As a matter of fact, in the largest series of thymomas so far, we collected 1,470 thymoma resections and we did not encounter a single case of metastasis to lymph nodes. Therefore, even though the TNM system may create a uniformity in tumor pathology, it does not apply to thymomas. In addition, in the interest of uniformity, this TNM system includes TX as primary tumor cannot be assessed—if it cannot be assessed then it may not be a thymoma, thus completely meaningless statement. Also, T0—no evidence of primary tumor—what does it mean pre or post treatment? Somewhere along the way a diagnosis of thymoma or thymic carcinoma should have been made. The actual meaning of T0 in the Moran staging system is for an encapsulated thymoma—meaning in situ tumor. Once again, this TNM system may be very useful for other types of malignancies but impractical at best when it is applied to thymoma. The T2 tumors—direct invasion into the pericardium either partial or full thickness. What do the authors mean by partial and full thickness? Which is their criterion for pericardial invasion? Where does the pericardial invasion starts for the authors? For the sake of clarity, the pericardium is a membrane which has two faces, one the mediastinal face and the other the cardiac face. Perhaps the authors need to review the Moran staging system, in which there is a clear definition of pericardial invasion;
- The N and M of this system has been addressed ad nauseum in different manuscripts stressing the important in thymic carcinoma but not for thymoma. Once a thymic carcinoma is metastatic to lymph nodes, it does not matter where the lymph node is, the impact on survival is different regardless of the location of the lymph node;
- M1 is defined by the authors as pleural, pericardial, or distant metastasis. What do the authors mean by distant metastasis? Do they mean below the diaphragm or above the thoracic inlet? (once again, the authors would have done well by reading Moran staging system). Do the authors consider that M1 disease of tumor in the pleura is equivalent to metastatic disease to the kidney? Obviously by placing those two possibilities into M1, that is what is implied. M1a and M1b do not occur at the time of diagnosis of thymoma in the overwhelming majority of cases. Thymomas invade by continuity and move along normal structures. Only when the tumor recurs is when one will observe separate nodules in adjacent structures. It appears that the inclusion of M disease is derived from conventional tumor pathology and not from actual facts on thymoma experience.
Table 1 provides a depiction of the different stages using the different schemas available. A closer view of this proposed AJCC schema clearly shows that it represents a clumsy adaptation of the two already proven schemas—Masaoka and Moran. However, there are several shortcomings about this schema. The N and M disease at the time of diagnosis of thymoma essentially will not take place so that the staging will be mainly based on T disease. Regarding T disease there is no mention whatsoever of the so-called drop metastasis that often occur in these tumors. The definition or the lack of it regarding pericardial invasion (T2) is ambiguous at best. The T3 and T4 definitions in terms of great vessels whether it is the aorta (T4) or the superior vena cava (T3) does not make sense for pathology staging. It has already been proven that once the tumor involves great vessels, it impacts survival. The problem with this adaptation of the AJCC is the lack of foresight in terms of limited disease and invasive disease, which impacts treatment options. It has been analyzed that stages 0 and I in the Moran schema correlate well with survival and that those patients will likely benefit from complete surgical resection only, while those with invasive disease may need additional treatment options. Therefore, the triaging of patients who need additional treatment should be the goal of any schema rather than just creating uniformity. In addition, what is more frightening is the adaptation of this system to thymic carcinoma. For instance, based on this AJCC schema, the authors consider that using this schema, a thymoma stage III A or B has similar clinical outcome as thymic carcinoma in the same stage. This is likely to be incorrect and attempting to created “uniformity” by having the same staging for thymoma and thymic carcinoma, by mixing these two tumors under the same umbrella is definitely not the way to clarify concepts. In addition, the issue of thymic carcinoma is more complex than it is stated in the guidelines. For instance, thymic carcinoma can be either squamous carcinoma, adenocarcinoma, or simply anaplastic carcinoma, and it has been shown that those tumors regardless of whether the tumor is limited to the mediastinum or not, the tumor may follow a more aggressive behavior (27). Needless to say, those proponents of this AJCC for thymoma and thymic carcinoma guidelines did not consider such possibilities.
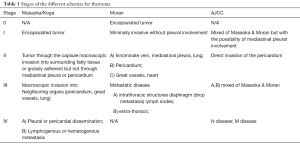
Full table
Comment
As with other publications on thymoma and thymic carcinoma, this new publication by the NCCN essentially has maintained previous misconceptions that do not help in the understanding of thymoma or thymic carcinoma. Where they should have stablished strict guidelines is in the diagnosis of thymic carcinoma or even in unusual variants of thymoma. However, the authors miss the opportunity as their focus was more in providing a table on obligatory and optional criteria for thymoma which is exactly where the efforts should not be. The WHO histological translator of thymoma has been proven that is meaningless. The classifications proposed by Bernatz, Marino-Muller-Hermelink, and Suster-Moran have the advantage that were real proposals based on actual review of cases and not derived from the thin air or to satisfy personal or political agendas. The introduction of a TNM schema for staging thymoma and mixing the same staging system with thymic carcinoma without hard data supporting such concept is imprecise at best. Nevertheless, it is important to mention for those who will be using this TNM system that the T is essentially a “borrowing” from the two schemas—Masaoka and Moran’ schema. The use of Masaoka or Moran’s proposal for staging thymomas is based on actual cases and not elaborated from the thin air. Attempting to adapt one or two schemas into a proposed TNM is not correct. All of the above brings us back to our initial assessment, and that is that unless a real panel of experts is put together to shed some light in this particular topic, the issue of thymoma will continue to be elusive and poorly understood. Guidelines are important as long as they represent hard data of series of actual cases and uniformity is good as long as it applies to the tumor in question. Otherwise, repeating the same erroneous concepts for scientific that they may look, does not make them correct. One would expect that any type of guidelines be stricter in terms of reviewing current analysis rather than just repeating what has been stated or adapting something that is just not based on hard data.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med .2018.07.05). CAM serves as an unpaid editorial board member of Mediastinum from Feb 2018 to Jan 2020. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bernatz PE, Harrison EG, Clagett OT. Thymoma: a clinicopathologic study. J Thorac Cardiovasc Surg 1961;42:424-44. [PubMed]
- Bernatz PE, Khonsari S, Harrison EG Jr, et al. Thymoma: factors influencing prognosis. Surg Clin North Am 1973;53:885-92. [Crossref] [PubMed]
- Salyer WR, Eggleston JC. Thymoma: a clinical and pathological study of 65 cases. Cancer 1976;37:229-49. [Crossref] [PubMed]
- Marino M, Müller-Hermelink HK. Thymoma and thymic carcinoma. Relation of thymoma epithelial cells to the cortical and medullary differentiation of thymus. Virchows Arch A Pathol Anat Histopathol 1985;407:119-49. [Crossref] [PubMed]
- Kirchner T, Schalke B, Buchwald J, et al. Well-differentiated thymic carcinoma. An organotypical low-grade carcinoma with relationship to cortical thymoma. Am J Surg Pathol 1992;16:1153-69. [Crossref] [PubMed]
- Kirchner T, Muller-Hermelin HK. New approaches to the diagnosis of thymic epithelial tumors. Prog Surg Pathol 1989;10:167-89. [Crossref]
- Moran CA, Kalhor N, Suster S. Invasive spindle cell thymomas (WHO Type A): a clinicopathologic correlation of 41 cases. Am J Clin Pathol 2010;134:793-8. [Crossref] [PubMed]
- Moran CA, Suster S. On the histologic heterogeneity of thymic epithelial neoplasms. Impact of sampling in subtyping and classification of thymomas. Am J Clin Pathol 2000;114:760-6. [Crossref] [PubMed]
- World Health Organization Ingternational Histological Classification of Tumours. Histological Typing of Tumours of the Thymus. Rosai J. In collaboration with L. H. Sobin and Pathologists in 8 countries. 2nd Edition. Berlin Heidelberg: Springer-Verlag 1999.
- Suster S, Moran CA. Thymoma, atypical thymoma, and thymic carcinoma. A novel conceptual approach to the classification of thymic epithelial neoplasms. Am J Clin Pathol 1999;111:826-33. [Crossref] [PubMed]
- Chalabreysse L, Etienne-Mastroianni B, Adeleine P, et al. Thymic carcinoma: a clinicopathological and immunohistological study of 19 cases. Histopathology 2004;44:367-74. [Crossref] [PubMed]
- Rieker RJ, Hoegel J, Morresi-Hauf A, et al. Histologic classification of thymic epithelial tumors: comparison of established classification schemes. Int J Cancer 2002;98:900-6. [Crossref] [PubMed]
- Moran CA, Weissferdt A, Kalhor N, et al. Thymomas I: a clinicopathologic correlation of 250 cases with emphasis on the World Health Organization schema. Am J Clin Pathol 2012;137:444-50. [Crossref] [PubMed]
- Weissferdt A, Kalhor N, Bishop JA, et al. Thymoma: a clinicopathological correlation of 1470 cases. Hum Pathol 2018;73:7-15. [Crossref] [PubMed]
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [Crossref] [PubMed]
- Koga K, Matsuno Y, Noguchi M, et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int 1994;44:359-67. [Crossref] [PubMed]
- Moran CA, Walsh G, Suster S, et al. Thymomas II: a clinicopathologic correlation of 250 cases with a proposed staging system with emphasis on pathologic assessment. Am J Clin Pathol 2012;137:451-61. [Crossref] [PubMed]
- Moran CA, Suster S. Thymic carcinoma: current concepts and histologic features. Hematol Oncol Clin North Am 2008;22:393-407. [Crossref] [PubMed]
- Gupta R, Marchevsky AM, McKenna RJ, et al. Evidence-based pathology and the pathologic evaluation of thymomas: transcapsular invasion is not a significant prognostic feature. Arch Pathol Lab Med 2008;132:926-30. [PubMed]
- Weissferdt A, Moran CA. Thymic carcinoma, part 1: a clinicopathologic and immunohistochemical study of 65 cases. Am J Clin Pathol 2012;138:103-14. [Crossref] [PubMed]
- Weissferdt A, Moran CA. Thymic carcinoma, part 2: a clinicopathologic correlation of 33 cases with a proposed staging system. Am J Clin Pathol 2012;138:115-21. [Crossref] [PubMed]
- National Comprehensive Cancer network. NCCN clinical practice guidelines in oncology (NCCN guidelines) Thymoma and Thymic carcinoma, version 2. 2018. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx
- Suster S, Moran CA. Micronodular thymoma with lymphoid B-cell hyperplasia: clinicopathologic and immunohistochemical study of eighteen cases of a distinctive morphologic variant of thymic epithelial neoplasm. Am J Surg Pathol 1999;23:955-62. [Crossref] [PubMed]
- Weissferdt A, Moran CA. Micronodular thymic carcinoma with lymphoid hyperplasia: a clinicopathological and immunohistochemical study of five cases. Mod Pathol 2012;25:993-9. [Crossref] [PubMed]
- Suster S, Moran CA, Chan JK. Thymoma with pseudosarcomatous stroma: report of an unusual histologic variant of thymic epithelial neoplasm that may simulate carcinosarcoma. Am J Surg Pathol 1997;21:1316-23. [Crossref] [PubMed]
- Yoneda S, Marx A, Heimann S, et al. Low-grade metaplastic carcinoma of the thymus. Histopathology 1999;35:19-30. [Crossref] [PubMed]
- Kalhor N, Moran CA. Primary thymic adenocarcinomas: a clinicopathological and immunohistochemical study of 16 cases with emphasis on the morphological spectrum of differentiation. Hum Pathol 2018;74:73-82. [Crossref] [PubMed]
Cite this article as: Kalhor N, Moran CA. Thymoma and thymic carcinoma: a perspective on the NCCN clinical practice guidelines in oncology. Mediastinum 2018;2:49.


