Video-assisted thoracic surgery thymectomy: a left-sided approach
Introduction
Traditionally thymectomies have been performed through open approaches such as sternotomy, thoracotomy or open transcervical technique. In the last decades, the minimally invasive surgery (MIS) has evolved and video-assisted thoracic surgery (VATS) or robotic-assisted thoracoscopic surgery (RATS) have become the standard approach for thymic disease.
Indications for MIS include thymectomy for the management of non-thymomatous myasthenia gravis (MG), benign thymic pathology and small and well encapsulated non-invasive tumours. MIS for more advanced cases has been described, however its indications remain controversial. Many surgeons are reluctant to adopt minimally invasive approaches because they concerned about whether an oncologically complete resection can be achieved; they considered that the risk of capsular disruption and tumour seedling of the pleural during surgical manipulation is higher through these approaches, which is associated with subsequent increased risk of local recurrence. However, with the growing experience, complex thymic tumours (large and invasive tumours, cystic thymomas, re-thymectomy in previous sternotomy and tumours following neoadjuvant treatment) have been resected in selected patients without compromising oncological radicality and patient safety (1-3).
On the other hand, it has been proved that VATS thymectomy is associated with low postoperative morbidity and mortality, less pain, better preserved pulmonary function, shorter hospital stays, better cosmetic results and equal outcomes compared to open surgery (4,5).
Another plus advantage of VATS approach is that, with the angles lens, it offers an excellent view of the thymus and mediastinum including all the structures and the fatty tissue between the diaphragm and the neck and it also provides magnification of the structures which can help in critical dissection. An added superiority of this approach is recognition of metastases not detected on preoperative radiological studies. So, we consider that the thoracoscopic approach provides better visualization than an open technique.
Despite these benefits, in Europe, according to the thymoma section of the European Society of Thoracic Surgeons (ESTS) annual database report 2017, 20.3% of thymic surgery was operated by VATS and 11.4% by RATS approach, whereas that 49.6% of cases were operated through a transsternal approach (6).
Several VATS approaches have been described and can be classified as unilateral (right or left), bilateral, subxiphoid, and bilateral VATS with cervical incision. Bilateral VATS thymectomy with and without cervical incision has been suggested to be better option than unilateral VATS thymectomy due to optimal evaluation of both chest cavities and cervical area; regardless, outcomes are equivalent when comparing both approaches (4). We describe the left approach, which is currently our preferred approach. The left sided approach allows an excellent visualization of the aortopulmonary window where some thymic tissue can be located. However, some surgeons prefer the right sided approach because, in this technique, the junction of the superior vena cava and innominate vein can be clearly recognized (7). We consider that this intersection is well identified with a left sided approach.
Patient selection and workup
VATS thymectomy is indicated in patients with non-thymomatous MG and well capsulated thymomas. In relation with the size of the tumor, some authors suggest that 5 cm is the limit to consider a thymoma resectable by VATS (8). If there is ever any question of invasion, the approach choice depends on which structures are involved. We routinely resect pericardium thoracoscopically. However, the tumor invades the innominate vein the approach is debatable. We consider that ligation and resection is achievable by VATS, but if we plan a reconstruction of the vein we prefer an open approach.
VATS thymectomy has some limitations such as obesity and cardiomegaly, which lead to a decreased maneuverability of instruments. However, it has few contraindications. On one hand, we have to consider general contraindications for surgery such as severe coagulopathy; and in the other hand, we have to take into consideration the specific contraindications of the procedure which include pleural adhesions after previous sternotomy—this is arguable—and patients with severe underlying lung disease who are unfit to allow single-lung ventilation (4).
Description of the approach (pre-operative preparation and equipment preference card)
Pre-operative preparation
In preparation for surgery, patients should have a preoperative blood test, an EKG and a computed tomography (CT) scan with intravenous contrast. In case of MG, a multidisciplinary team (patient´s neurologist, surgeon and anesthesiologist) should plan the surgical process and discuss about the need for peri-operative intravenous immunoglobulin therapy. During the all the perioperative period, patients should continue their anti-cholinesterase inhibitors and corticosteroids.
Equipment preference card
10 mm 30 Degree camera; one 10 mm port and two 5 mm ports; one 5 mm endoscopic grasper; 5 mm Endoscopic Sucker; Electrocautery and/or 5 mm Maryland LigaSureTM (Medtronic, Minneapolis, MN, USA); 10 mm Endoscopic Retrieval Bag; Insufflation tube for CO2.
Procedure
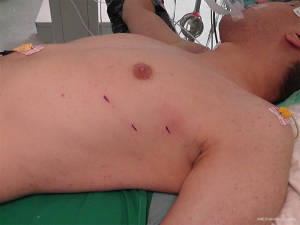
The patient is placed in a 30-degree semi-supine posture, with the left side of the chest raised. We recommend to put a pillow under the left hemithorax (Figure 1). The ipsilateral arm is placed on the surgical table in a natural and comfortable position just below the chest wall to obtain the best exposure of the left upper intercostal spaces. The right arm is held extended on a padded board. Both hemithorax must be included in the surgical field just in case a right approach is also required.
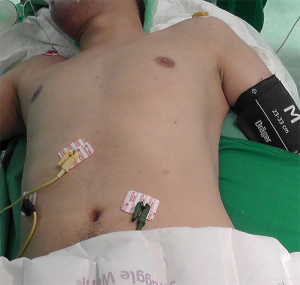
A double-lumen endotracheal tube is recommended, lung isolation contributes to a better exposure of the mediastinal space. Carbon dioxide insufflation (5–8 mmHg pressure at 4 L/min flow rate) helps to lung collapse, facilitate visualization and exposure of the thymus opening the cervical space for an excellent visualization of the superior horns.
We perform the VATS procedure through three ports. We have standardized our technique and we introduce the thoracoscope through a 10-mm port in the fifth intercostal space at the anterior axillary line. Under direct vision, two 5-mm instruments ports are placed: one in the third intercostal space at the anterior axillary line and the other in the fifth intercostal space along the infra-mammary fold (Figures 2,3). The insufflation tube for CO2 is connected to the 10mm port and we also introduce the 30º camera through this port. The two 5mm ports are used for instruments (ultrasonic device and grasper) (Figure 4). Generally, we use the upper port to dissect the thymus with the energy device (Maryland LigaSureTM) and the lower port to mobilize the thymus with the graspers. Occasionally we can change these instruments to get a better visualization and dissection of the gland.
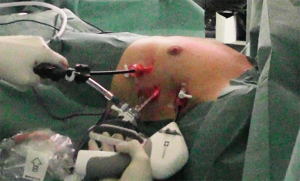
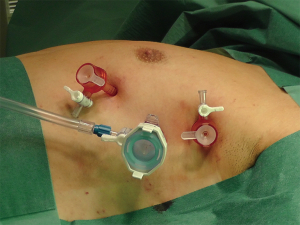
Figure 5: the second step consists in identifying the left phrenic nerve, sometimes retraction of the lung is needed. If there is a tumor, we recommend and starting the dissection away from it and dissecting it last; care must be taken to keep the plane of dissection beyond the capsule, to avoid direct traction to the capsule and to limit capsular tears and local and pleural contamination by tumoral cells. We start the dissection in the pleura behind the sternum and the dissection plane is extended cranially and inferiorly towards the diaphragm. Electrocautery and LigaSureTM (Medtronic) are the tools we use for dissection. Electrocautery is more precise and we use it to dissect around vessels. The Maryland LigaSureTM is useful for sealing and for dividing small vessels. Using either of them, the mediastinal pleura is dissected considering the landmark of the phrenic nerve. Care is taken to avoid injury of the nerve. Then we continue the dissection superiorly up to the internal mammary vessels. After that, the superior mediastinum is dissected transversely, trying to always stay anterior to the innominate vein. Then we go forward the right side as far laterally as visualization allows and at least until we visualize the point of convergence of the of the innominate vein and the superior vena cava. The dissection then proceeds lateral to medial, along the inferior border at the pericardial reflection. Once the right pleura and right phrenic nerve are identified, we continue the dissection cranially, always aligned with the nerve. If needed, the right pleura can be opened to better recognition of the right phrenic nerve. The thymus is then mobilized respecting pericardium and innominate vein. The thymic veins draining to the innominate vein are dissected and then clipped and divided using ultrasonic device or scissors. The right and the left thymic horns can be followed up from the chest and easily dissected into the neck combining traction and dissection below the gland and working all sides of the horn to deliver the cervical portion into the chest cavity. In patients with MG, complete radical thymectomy is achieved by en-bloc removal of the thymus, perithymic fat and tissue from the thoracic inlet to the diaphragm, and from phrenic nerve to phrenic nerve. The gland is removed in a plastic retrieval bag through the 10-mm port. To finish, we usually place a chest tube (16–20 Fr) in the anterior mediastinum.

Tips, trick and pitfalls
Trocar placement and visualization
- Pitfall: deficient visualization and maneuverability problems.
- Tips: Uni pulmonary ventilation and insufflation of the hemithorax with CO2 can significantly increase working space. Long trocars are needed in obese patients. Trocars must be placed away from each other.
- Pitfall: pain, intercostal nerve injury.
- Tips: we use a 5–10 mm ports to minimize intercostal nerve injury. Placement of the trocars under thoracoscopic visualization decreased the risk of complications.
- Tricks: it is useful to follow the landmark of the major pectoralis muscle or the inframammary fold to place the trocars.
Maneuverability and thymus mobilization
- Pitfall: excessive fatty tissue.
- Tips: a fourth medial 5mm port can help to increase the exposure and facilitate mobilization if necessary especially in large glands with abundant perithymic fat or obese patients. Longer instruments such as Maryland LigaSureTM 37 cm are available if required, although this situation is rare.
Phrenic nerves injury
- Pitfall: injury left the phrenic nerve.
- Tips: while dissecting the pleura near diaphragm, we avoid the use of electrocautery to prevent thermal injury of the nerve. We recommend using an energy device in this area to minimize the risk of thermal injury. It can be also useful to keep the perspective and the location of the nerve just modifying the direction of the camera and reevaluating the surgical field.
- Pitfall: injury of the right phrenic nerve.
- Tips: the key is making a dissection under direct vision. 30-degree camera can be useful to recognize the course of the nerve in the right side during a left sided approach. If there is any difficulty to identify the nerve, the right pleura can be opened facilitating its visualization.
Dissection of the superior horns
- Pitfall: injury of the innominate vein or bleeding of the thymic veins.
- Tips: the dissection along the innominate vein should be performed carefully with a perfect visualization. The key to prevent bleeding complications is to identify the innominate vein which sometimes is not easily observable. If any time, the visualization is not perfect, we recommend not to seal the thymic or fatty tissue because we are at risk of injuring any vessel. In case of intraoperative hemorrhage coming from the innominate vein, conversion to sternotomy or thoracotomy can be necessary. Thymic veins should be dissected with caution and sealed or clipped in case of large veins.
- Tricks: if conversion is needed, the 30-degree semi-supine position facilitate it. Surgical field should be also prepared for this emergency.
- Pitfall: opening the right pleura.
- Tips: this circumstance can lead to hypoxia or hypertension. In this case, decrease the insufflation pressure or increase the peak airway pressure must be necessary to maintain adequate oxygenation and ventilation. One chest tube placed in both hemithoraces and anterior mediastinum is sufficient to drain both pleural spaces.
- Pitfall: difficulties to dissect the cervical horns.
- Tips: CO2 insufflation helps to open the tissue planes in the cervical region. Directing the 30-degrees camera upward the thoracic inlet can be useful during this maneuver. When progress is not being easily made, modifying the perspective of the surgical field and redirect the dissection to a different anatomic area can help. Sometimes it can be useful changing the entrance port of the different instruments; introducing the energy device through the inframammary fold and the endoscopic grasper through the upper port can facilitate the dissection. A fourth 5 mm port placed medially allows a more direct access to this area.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors Nuria Novoa and Wentao Fang for the series “Minimally Invasive Thymectomy” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2018.03.16). The series “Minimally Invasive Thymectomy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vyas S, Agasthian T, Goh MH, et al. Thoracoscopic thymectomy in a previous sternotomy. Asian Cardiovasc Thorac Ann 2006;14:e108-10. [Crossref] [PubMed]
- Agasthian T, Lin SJ. Clinical outcome of video-assisted thymectomy for myasthenia gravis and thymoma. Asian Cardiovasc Thorac Ann 2010;18:234-9. [Crossref] [PubMed]
- Agasthian T. Can invasive thymomas be resected by video-assisted thoracoscopic surgery? Asian Cardiovasc Thorac Ann 2011;19:225-7. [Crossref] [PubMed]
- Ng CS, Wan IY, Yim AP. Video-assisted thoracic surgery thymectomy: the better approach. Ann Thorac Surg 2010;89:S2135-41. [Crossref] [PubMed]
- Rückert JC, Walter M, Müller JM. Pulmonary function after thoracoscopic thymectomy versus median sternotomy for myasthenia gravis. Ann Thorac Surg 2000;70:1656-61. [Crossref] [PubMed]
- ESTS Database annual report 2017. Available online: http://www.ests.org/private/database_reports.aspx
- Yim AP. Paradigm shift in surgical approaches to thymectomy. ANZ J Surg 2002;72:40-5. [Crossref] [PubMed]
- Ye B, Tantai JC, Ge XX, et al. Surgical techniques for early-stage thymoma: video-assisted thoracoscopic thymectomy versus transsternal thymectomy. J Thorac Cardiovasc Surg 2014;147:1599-603. [Crossref] [PubMed]
- Jimenez M, Gomez-Hernandez MT. Video of the procedure: dissection, identification of the anatomy and resection of the thymus. Asvide 2018;5:388. Available online: http://www.asvide.com/article/view/24128
Cite this article as: Jimenez M, Gomez-Hernandez MT. Video-assisted thoracic surgery thymectomy: a left-sided approach. Mediastinum 2018;2:29.