
Endobronchial ultrasound-guided transbronchial mediastinal cryobiopsy: a narrative review
Introduction
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is a pivotal tool for assessing mediastinal lymphadenopathy and is the method of choice for staging lung cancer. This minimally invasive procedure is safe and is often performed as an outpatient procedure.
Although it offers high diagnostic yield for primary pulmonary malignancy, at 90% (1), its efficacy for diagnosing lymphoma is debated (2), with a sensitivity of 65% (3). Similarly, its accuracy for identifying benign lesions, such as sarcoidosis (84%) and tuberculosis (80%), is less than ideal (4,5). Efforts to enhance these yields have embraced the use of diverse needle sizes, core biopsy needles, and intranodal forceps biopsy (6-8); however, the absence of histological information remains a significant challenge.
A promising alternative emerges in the form of EBUS-guided transbronchial mediastinal cryobiopsy (EBUS-TMC). This innovative technique shows promise for histological diagnoses in primary pulmonary malignancies and lymphoma and exhibits superior diagnostic capabilities for benign lesions. In this narrative review, we assess the diagnostic accuracy and safety of EBUS-TMC for diagnosing malignant lesions and its proficiency in identifying benign conditions such as tuberculosis and sarcoidosis. In addition, we address the technical nuances of EBUS-TMC, drawing on the existing body of literature. This article is presented in accordance with the Narrative Review reporting checklist (available at https://med.amegroups.com/article/view/10.21037/med-23-34/rc).
Methods
We conducted a comprehensive literature search of the PubMed® database using a predefined protocol. Our search terms included (“endobronchial ultrasound” OR “endobronchial ultrasonography” OR “EBUS” OR “endobronchial ultrasound-guided”) AND (“TBNC” OR “transbronchial nodal cryobiopsy” OR “mediastinal cryobiopsy” OR “TMC”). We restricted our search to articles in English up to July 1, 2023. We also manually explored bibliographic references from the chosen papers to capture any additional pertinent studies.
We first identified potentially relevant papers by scrutinizing the abstracts procured from our search. Then we shortlisted these based on predetermined criteria. The review encompassed original articles and case series in English, prioritizing clinical trials. We excluded case reports and conference abstracts (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | 25.05.2023–01.07.2023 |
| Databases and other sources searched | PubMed |
| Search terms used | (“Endobronchial ultrasound” OR “endobronchial ultrasonography” OR “EBUS” OR “endobronchial ultrasound-guided”) AND (“TBNC” OR “transbronchial nodal cryobiopsy” OR “mediastinal cryobiopsy” OR “TMC”) |
| Timeframe | From inception to 01.07.2023 |
| Inclusion and exclusion criteria | Inclusion criteria: original articles and case series written in English |
| Exclusion criteria: case reports, conference abstracts | |
| Selection process | One author generated a list of prospective studies, and the second author assessed them to verify their suitability |
EBUS, endobronchial ultrasound; TBNC, transbronchial nodal cryobiopsy; TMC, transbronchial mediastinal cryobiopsy.
Studies
Our review included seven publications, including two significant multicenter randomized control studies by the same research team that recruited patients from China and Germany (Table 2). Zhang et al. (9) studied 197 patients randomized into two groups, either to undergo EBUS-TBNA followed by cryobiopsy or the inverse. Fan et al. (10) conducted an open-label, randomized trial of 297 participants from three hospitals (two in China and one in Germany); the patients underwent either EBUS-TBNA followed by mediastinal cryobiopsy or exclusively EBUS-TBNA.
Table 2
| Study | Year | Study design | N | Diagnostic yield of TBNA (%) | Diagnostic yield of TMC (%) | Tools used to create a tract | Cryoprobe activation time (s) | Sedation | Airway management device | Adverse events |
|---|---|---|---|---|---|---|---|---|---|---|
| Zhang (9) | 2021 | RCT | 197 | 79.9 | 91.8 | High frequency needle knife | 7 | Conscious sedation | Nil | 20 bleeding, 2 PTX, 1 PNM |
| Fan (10) | 2023 | RCT | 271 | 82 | 94% (TBNA + TMC) | High frequency needle knife | 7 | Conscious sedation | Nil | 5 bleeding, 6 PTX, 1 PNM |
| Ariza-Prota (11) | 2023 | Prospective | 50 | 82 | 96 | 22-G TBNA needle | 4 | Conscious sedation | Nil | Bleeding (number not mentioned) |
| Maturu (12) | 2023 | Prospective | 46 | 41.3 | 71.7 | 19-G TBNA needle | 5 to 6 | General anesthesia | LMA | 14 bleeding |
| Gershman (13) | 2022 | Prospective | 24 | 87.5 | 83.3 | Procore 22-G TBNA needle [16], Nd:YAG laser [8] | 3 to 4 | Deep sedation | LMA | Nil |
| Gonuguntla (14) | 2021 | Case series | 4 | 75 | 100 | 19-, 21-, and 22-G TBNA needles | 3 | General anesthesia | LMA | 1 bleeding |
| Genova (15) | 2022 | Case series | 5 | 80 | 40 | 19-G TBNA needle | 4 | Deep sedation | Nil | Nil |
EBUS-TMC, endobronchial ultrasound-guided transbronchial mediastinal cryobiopsy; TBNA, transbronchial needle aspiration; RCT, randomized controlled trial; PTX, pneumothorax; PNM, pneumomediastinum; LMA, laryngeal mask airway.
Ariza-Prota et al. (11) conducted a prospective study in Spain on 50 patients, wherein participants underwent EBUS-TBNA followed by EBUS-TMC. Maturu et al. (12) carried out a prospective study on 46 patients in India, in which EBUS-TMC was conducted if rapid on-site evaluation (ROSE) during EBUS-TBNA yielded insufficient or inconclusive results. A prospective study in Israel documented EBUS-TMC in 24 patients (13) and two case reviews have been conducted, one on four patients (14) and one on five patients (15).
Diagnostic yield in malignancy
In Zhang et al. (9), the overall diagnostic yield was 79.9% for EBUS-TBNA vs. 91.8% for EBUS-TMC (P=0.001). However, the two techniques displayed similar diagnostic yields for common lung cancers (94.1% for EBUS-TBNA vs. 95.6% for EBUS-TMC). For rarer tumors, EBUS-TMC exhibited a notably higher yield (91.7% compared to 25.0% for EBUS-TBNA, P=0.001). Cryobiopsy was notably more adept for diagnosing and subclassifying lymphomas, outstripping TBNA. A case of lymphoma necessitated cervical mediastinoscopy for diagnosis when both procedures were inconclusive.
In Fan et al. (10), cryobiopsy notably augmented the overall diagnostic yield for mediastinal lesions. However, subgroup analyses demonstrated similar diagnostic yields for mediastinal metastases in both groups, with 68 (99%) of the 69 participants in each group having similar results [relative risk (RR), 1.00; 95% confidence interval (CI): 0.96–1.04; P>0.99]. For rarer tumors, there was no notable diagnostic difference. In lymphoma patients, a nonsignificant enhancement in diagnostic yield with cryobiopsy was observed: 8 (80%) of 10 vs. 5 (50%) of 10, with a RR of 0.63 (95% CI: 0.31–1.25). However, all diagnosed instances were effectively subclassified, thereby surpassing EBUS-TBNA alone: 1 (20%) of 5 participants vs. 8 (100%) of 8, RR, 5.00 (95% CI: 0.87–28.86).
In Ariza-Prota et al. (11), EBUS-TMC identified six lung adenocarcinoma and four squamous cell lung carcinoma cases, which were previously classified as inconclusive or inaccurately classified by EBUS-TBNA. For small cell lung carcinoma, the two methods displayed comparable yields. In addition, EBUS-TMC achieved a 100% diagnostic rate for suspected lymphoma cases, accurately subclassifying them, while EBUS-TBNA failed to provide a definitive lymphoma diagnosis.
In Maturu et al. (12), EBUS-TMC confirmed diagnoses in 33 cases (71.7%). In 14 cases with inadequate ROSE that subsequently underwent EBUS-TMC, malignancy was confirmed by both EBUS-TMC and EBUS-TBNA. Notably, EBUS-TMC diagnosed two lymphoma and two sarcoma cases, which EBUS-TBNA did not.
In Gershman et al. (13), cryobiopsy provided a diagnosis in 20 cases (83.3%), while TBNA alone diagnosed 21 patients (87.5%). Of these, 13 had malignancies. Yet, in a single case, both methods failed to provide a definitive diagnosis of adenocarcinoma.
In Gonuguntla et al. (14) both methods detected adenocarcinoma one of four patients. In another patient with a malignancy, EBUS-TBNA only detected atypical cells whereas EBUS-TMC later confirmed metastatic adenocarcinoma originating from the breast.
In Genova et al. (15), EBUS-TMC offered added diagnostic value over EBUS-TBNA in specific cases. For instance, it enabled a final diagnosis of squamous cell carcinoma in a non-small cell lung cancer (NSCLC) patient and provided better histologic characterization for a lymphoma patient. Nonetheless, there were instances where it did not detect neoplastic cells in a small cell lung cancer patient, despite detection by EBUS-TBNA.
In conclusion, EBUS-TMC offers a diagnostic yield similar to EBUS-TBNA for primary pulmonary malignancies but stands out in diagnosing and subclassifying lymphoma.
Diagnostic yield in benign conditions
Several studies have reported better results for EBUS-TMC over EBUS-TBNA. One study reported diagnostic yields of 80.9% vs. 53.2%, respectively (9). Notably, in that study, EBUS-TMC accurately diagnosed all instances of sarcoidosis and tuberculosis (where EBUS-TBNA had lower diagnostic rates for these conditions. Another study reported that pairing these two methods resulted in superior sensitivity (compared to regular needle aspiration) for diagnosing benign disorders, with rates of 94% vs. 67% (10). Further intra-individual subgroup analyses indicated significantly enhanced diagnostic precision for benign conditions after adding cryobiopsy to standard EBUS-TBNA, as opposed to using EBUS-TBNA alone [45 (94%) of 48 participants vs. 32 (67%) of 48; RR, 1.41; 95% CI: 1.14–1.74; P=0.0009]. For those subjected to both procedures, EBUS-TMC unerringly diagnosed all instances of sarcoidosis (n=16) and tuberculosis (n=19). In comparison, EBUS-TBNA identified only 12 cases (9%) of sarcoidosis and 11 cases (8%) of tuberculosis. In another work, the diagnostic yields of EBUS-TBNA and EBUS-TMC for sarcoidosis patients showed no significant differences (11). Nevertheless, EBUS-TMC identified four anthracosis cases that EBUS-TBNA overlooked. In Maturu et al. (12), out of 27 cases with ambiguous EBUS-TBNA outcomes, EBUS-TMC discerned 14, including 10 benign cases (six tuberculosis, three sarcoidosis, and one fungal infection).
However, the other studies in our sample reported similar (or ambiguous) results between the two methods. In one, both methods registered positive outcomes for all sarcoidosis patients (13); in another, both diagnostic techniques were adept at diagnosing one instance each of sarcoidosis and tuberculosis (14); and in the final study, anthracosis was diagnosed in one patient via TBNA but cryobiopsy failed to provide adequate material for evaluation (15).
In sum, these studies indicate that, for benign lesions, EBUS-TMC typically (but not always) boasts a superior diagnostic yield over TBNA.
Suitability of EBUS-TMC samples for molecular and immunological studies
The primary advantage of EBUS-TMC lies in its ability to procure larger tissue samples, preserving the architecture of tissue samples. This is crucial for molecular and immunological assessments of malignancy cases. As NSCLC biomarkers grow in number, the demand for tissue volume for comprehensive tests increases, particularly with the increasing adoption of next-generation sequencing. Providing high-quality tissue during initial diagnosis can lessen the demand for subsequent procedures.
Zhang et al. (9) revealed that EBUS-TMC facilitated richer pathological details and enhanced immunohistochemistry staining, capabilities that were limited with TBNA samples. In cases of NSCLC, 93.3% of cryobiopsy materials were suitable for gene mutation PCR testing, a stark contrast to the only 73.5% of TBNA samples that were suitable (9). In Fan et al. (10), which combined the two methods, genomic testing for lung cancer and PD-L1 immunohistochemistry assays were viable for nearly 97% of NSCLC samples (37 of 38); when using only EBUS-TBNA, these tests were feasible in 79% (30 of 38) of samples. Thus, the dual approach significantly enhanced the available samples for testing.
Ariza-Prota et al. (11) demonstrated that cryobiopsy was pivotal for optimal molecular testing when EBUS-TBNA fell short in delivering a conclusive lung cancer diagnosis. In Maturu et al. (12) for all 14 cases of malignancy with inadequate ROSE who underwent EBUS-TMC, the cryobiopsy material was adequate for ancillary studies in all cases, while the cellblock obtained from additional TBNA passes had adequate material for ancillary studies in 5 of 14 cases (35.7%). Gershman et al. (13) did not specify the suitability of samples for auxiliary tests (13), Gonuguntla et al. (14) underscored the adequacy of cryobiopsy for immunohistochemistry (14), and Genova et al. (15) reported the superiority of EBUS-TMC, emphasizing its provision of sample tissue for detailed histologic diagnosis and subsequent molecular detailing in malignant cases.
Safety
The act of sampling extensive tissue through EBUS-TMC is not without its inherent risks, including potential bleeding, pneumothorax, pneumomediastinum, and even mediastinitis. However, very few complications have been reported. Zhang et al. (9) reported no major complications, noting only minimal bleeding in 20 subjects, which naturally subsided. Both pneumothorax and pneumomediastinum appeared in a small number of cases (1.0% and 0.5%, respectively), and these too dissipated on their own. Similarly, in Fan et al. (10), complication rates were low for all treatment methods. Only a fraction of the participant pool experienced complications; these included six pneumothorax incidents and a single pneumomediastinum case and none of them required intervention. They also reported 1–2% grade 3–4 airway bleeding. The remaining studies reported an absence of major complications, during both the procedures and follow-up (11-15). These data suggest that EBUS-TMC has a commendable safety record with sparse adverse outcomes.
Technical aspects
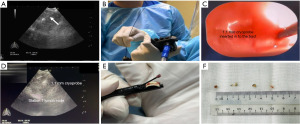
EBUS-TMC employs a cryoprobe (ERBE, Medizintechnik, Tübingen, Germany) that is introduced through the working channel of the EBUS scope (Figure 1). The tip of the cryoprobe is then inserted through the puncture site created by the EBUS-TBNA needle. In some instances, tools such as an electrocautery knife or laser are used to widen the tract, facilitating insertion of the cryoprobe. Furthermore, this tract facilitates passage of the biopsy specimen during retrieval of the cryoprobe and bronchoscope post EBUS-TMC. Then EBUS imaging is used to confirm the cryoprobe position within the node. Doppler imaging is used to help identify any intervening vessels. Once the cryoprobe placement is verified, it is activated for a duration ranging from 3 to 10 seconds. Following this, the EBUS scope, with the attached cryoprobe, is extracted. The retrieved specimen is thawed in saline before being preserved in a formalin-filled specimen pot. Post-extraction, the EBUS scope is immediately used to inspect the puncture site for possible bleeding.
Sedation
The three most comprehensive studies on EBUS-TMC (9-11) performed the procedure with patients under conscious sedation. However, some studies preferred using general anesthesia, with a laryngeal mask airway employed for managing the airway.
Creating a tract for cryobiopsy
There can be challenges inserting the cryoprobe through the tract initially made by the EBUS-TBNA needle. To make insertion smoother, extra tools can be used to widen the tract. Two of the studies (9,10) used a high-frequency needle-knife to make a small slit in the tracheobronchial wall near the lesion, which makes cryoprobe insertion easier (using Olympus KD-31C-1, Olympus, Tokyo, Japan). Another one (11) inserted the cryoprobe through the tract formed after making three passes with a 22-G EBUS-TBNA needle. In instances of probable silicosis (five cases), five passes were necessary for the cryoprobe to breach the bronchial wall. When faced with challenges inserting the cryoprobe through the tract created by the TBNA needles (8 out of 24 patients), Gershman et al. (13) used an Nd:YAG laser (Medialis Fiberton 8100, Dornier MedTech, Singapore, Singapore) to enlarge the tract. In these instances, a 1.7-mm cryoprobe was used to perform EBUS-TMC procedures. The remaining three studies (12,14,15) used the tract formed by a 19-G EBUS-TBNA needle for cryoprobe insertion.
Nodes biopsied
Among the studies included in this review, EBUS-TMC was mainly carried out in the mediastinum, notably at stations 4R and 7 (9-12,14,15); hilar lymph nodes were also accessed, particularly at stations 10R and 11R (9,10) or 11L (11,12). Gershman et al. (13) mainly sampled station 7 (n=26), with only a single instance at station 4L.
Procedure duration
Most studies (12-15) did not report the time it took to perform EBUS-TMC in addition to the standard EBUS-TBNA procedure. Among those that did, the time to perform EBUS-TBNA vs. EBUS-TMC differed, with the cryobiopsy generally being lengthier than TBNA. Zhang et al. (9) noted a mean time of 31.9±9.1 minutes for EBUS examination, with cryobiopsy taking slightly longer than TBNA (11.7±5.3 vs. 9.4±2.6 minutes, P<0.001). Fan et al. (10) reported a mean time of 22.3 min for combined EBUS-TBNA and EBUS-TMC, slightly exceeding EBUS-TBNA alone (17.0 minutes; P<0.0001). The final study to report procedure duration (11) did not compare the procedure time between EBUS-TBNA and EBUS-TMC, but reported an average time of 33.96±5.86 minutes for the total procedure (EBUS-TBNA and EBUS-TMC), with EBUS-TMC taking 9.54±2.10 minutes.
Cryoprobe activation time and number of cryobiopsies
The ideal cryoprobe activation time and the necessary number of cryobiopsies to ensure adequate sample size remain undefined. Among the considered studies, the activation times generally ranged from 3 to 7 seconds (9-15). The number of cryobiopsies conducted on each node ranged between 1 and 4, highlighting the need for more research and standardization to pinpoint optimal practices for the procedure.
Generalizability of the results
The ability to generalize the results is constrained by notable variation among studies. First, there was significant heterogeneity in design among the studies. Only two (9,10) were large, randomized trials, with just one of those comparing two groups (EBUS-TBNA vs. EBUS-TBNA and EBUS-TMC) (10). Both were conducted by the same team. This may reflect that team’s extensive proficiency with the procedure but also suggests that less-seasoned teams could yield results indicating less effectiveness and more complications. There was also variation in technical aspects among our sample studies, such as cryoprobe activation duration and the number of cryobiopsy samples taken. Such differences could differentially affect diagnostic outcomes and potential complications. Extended freezing durations or multiple biopsies could affect the procedure’s overall safety and efficacy.
Limitations
Given that EBUS-TMC is a novel technique, there have been few studies on the subject to date, and its diagnostic precision and safety are not yet fully elucidated. This underscores the need for more expansive research and larger-scale studies to thoroughly assess these factors in relation to the diagnosis of both malignant and benign conditions tied to mediastinal lymphadenopathy. Moreover, the studies included in this review differ in their designs, sample sizes, and technical elements, complicating direct comparisons of their findings.
Discussion
The studies explored in this narrative review suggest that EBUS-TMC can be used to obtain adequate diagnostic tissue for both malignant and benign conditions while maintaining a commendable safety profile. Its main strength is the ability to secure large tissue samples without compromising the tissue’s architecture. This makes it particularly pertinent for lymphoproliferative disorders, benign conditions, and rare tumors, which often necessitate histologic samples to evaluate the overall tissue structure. Although this may not be pivotal for primary pulmonary malignancies where EBUS-TBNA excels in terms of diagnostic capability, the escalating demand for in-depth molecular and immunological testing, particularly in NSCLC scenarios, highlights the significance of EBUS-TMC.
However, there are notable limitations to integrating EBUS-TMC with EBUS-TBNA. Foremost, it would elevate the procedure’s cost, primarily due to the need to use supplementary equipment such as a cryosurgical unit and one-time-use cryoprobes. It would also increase overall procedural duration. For multiple cryobiopsy specimens, the EBUS scope must be withdrawn and reinserted to relocate nodes. Challenges may also arise when attempting to insert the cryoprobe through the tract created by the TBNA needle, particularly when encountering a stiff bronchial wall. This might necessitate additional instruments, extending the procedure. The decreasing availability of certain tools further complicates the procedure and may require transitioning to bronchoscopes with larger working channels, adding to the procedure’s length.
Another concern is the potential inaccuracy of EBUS-TMC for sampling various lymph node areas, compared to the multidirectional ‘fanning’ technique offered by EBUS-TBNA. Adjusting the cryoprobe penetration depth in different areas of the lymph node can be challenging, particularly with smaller nodes. This limitation was evident in reviewed studies where EBUS-TMC failed to diagnose specific instances of lung cancer, a shortcoming not seen with EBUS-TBNA.
Furthermore, acquiring larger samples via EBUS-TMC poses risks such as bleeding, pneumothorax, pneumomediastinum, and the potential for mediastinitis.
Given these constraints, using EBUS-TMC as a primary diagnostic approach alongside EBUS-TBNA might not be suitable for all patients. Furthermore, it is imperative to recognize instances where EBUS-TMC could miss certain diagnoses that EBUS-TBNA might detect. Maturu et al. (12) presented a diagnostic algorithm to identify patients who might benefit from concurrent EBUS-TMC and EBUS-TBNA use, particularly when ROSE produces inconclusive results.
Future EBUS-TMC research should concentrate on determining the ideal cryoprobe activation duration and the precise number of samples needed for accurate diagnosis and additional examinations. It is vital to carry out larger studies to ascertain the validity of merging EBUS-TMC with EBUS-TBNA and pinpoint the specific scenarios where it is most advantageous. Randomized controlled trials that compare diverse specimen collection techniques from mediastinal nodes are essential. Such trials should assess not only diagnostic yield but also the adequacy of acquired material for molecular and immunological evaluations. Addressing these facets will enhance our comprehension of the potential benefits of EBUS-TMC and bolster diagnostic precision for mediastinal lymphadenopathy.
Conclusions
EBUS-TMC is a promising technique for procuring diagnostic tissue for both malignant and benign conditions. However, uncertainties surrounding appropriate case selection and technical nuances necessitate further comprehensive research.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://med.amegroups.com/article/view/10.21037/med-23-34/rc
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-23-34/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-23-34/coif). M.O. reports receiving speaker fees from Olympus Corporatio and AMCO Inc. as a guest speaker at academic medical meetings. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre M, Reda M, Musso V, et al. Diagnostic accuracy of endobronchial ultrasound-transbronchial needle aspiration (EBUS-TBNA) for mediastinal lymph node staging of lung cancer. Mediastinum 2021;5:15. [Crossref] [PubMed]
- Du Rand IA, Barber PV, Goldring J, et al. British Thoracic Society guideline for advanced diagnostic and therapeutic flexible bronchoscopy in adults. Thorax 2011;66:iii1-21. [Crossref] [PubMed]
- Erer OF, Erol S, Anar C, et al. Diagnostic yield of EBUS-TBNA for lymphoma and review of the literature. Endosc Ultrasound 2017;6:317-22. [Crossref] [PubMed]
- Trisolini R, Lazzari Agli L, Tinelli C, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for diagnosis of sarcoidosis in clinically unselected study populations. Respirology 2015;20:226-34. [Crossref] [PubMed]
- Ye W, Zhang R, Xu X, et al. Diagnostic Efficacy and Safety of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration in Intrathoracic Tuberculosis: A Meta-analysis. J Ultrasound Med 2015;34:1645-50. [Crossref] [PubMed]
- Franke KJ, Bruckner C, Szyrach M, et al. The contribution of endobronchial ultrasound-guided forceps biopsy in the diagnostic workup of unexplained mediastinal and hilar lymphadenopathy. Lung 2012;190:227-32. [Crossref] [PubMed]
- Herth FJ, Morgan RK, Eberhardt R, et al. Endobronchial ultrasound-guided miniforceps biopsy in the biopsy of subcarinal masses in patients with low likelihood of non-small cell lung cancer. Ann Thorac Surg 2008;85:1874-8. [Crossref] [PubMed]
- McCracken DJ, Bailey M, McDermott MT, et al. A retrospective analysis comparing the use of ProCore® with standard fine needle aspiration in endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA). Ir J Med Sci 2019;188:85-8. [Crossref] [PubMed]
- Zhang J, Guo JR, Huang ZS, et al. Transbronchial mediastinal cryobiopsy in the diagnosis of mediastinal lesions: a randomised trial. Eur Respir J 2021;58:2100055. [Crossref] [PubMed]
- Fan Y, Zhang AM, Wu XL, et al. Transbronchial needle aspiration combined with cryobiopsy in the diagnosis of mediastinal diseases: a multicentre, open-label, randomised trial. Lancet Respir Med 2023;11:256-64. [Crossref] [PubMed]
- Ariza-Prota M, Pérez-Pallarés J, Fernández-Fernández A, et al. Endobronchial ultrasound-guided transbronchial mediastinal cryobiopsy in the diagnosis of mediastinal lesions: safety, feasibility and diagnostic yield - experience in 50 cases. ERJ Open Res 2023;9:00448-2022. [Crossref] [PubMed]
- Maturu VN, Prasad VP, Vaddepally CR, et al. Endobronchial Ultrasound-guided Mediastinal Lymph Nodal Cryobiopsy in Patients With Nondiagnostic/Inadequate Rapid On-site Evaluation: A New Step in the Diagnostic Algorithm. J Bronchology Interv Pulmonol 2024;31:2-12. [Crossref] [PubMed]
- Gershman E, Amram Ikan A, Pertzov B, et al. Mediastinal "deep freeze"-transcarinal lymph node cryobiopsy. Thorac Cancer 2022;13:1592-6. [Crossref] [PubMed]
- Gonuguntla HK, Shah M, Gupta N, et al. Endobronchial ultrasound-guided transbronchial cryo-nodal biopsy: a novel approach for mediastinal lymph node sampling. Respirol Case Rep 2021;9:e00808. [Crossref] [PubMed]
- Genova C, Tagliabue E, Mora M, et al. Potential application of cryobiopsy for histo-molecular characterization of mediastinal lymph nodes in patients with thoracic malignancies: a case presentation series and implications for future developments. BMC Pulm Med 2022;22:5. [Crossref] [PubMed]
Cite this article as: Ramarmuty HY, Oki M. Endobronchial ultrasound-guided transbronchial mediastinal cryobiopsy: a narrative review. Mediastinum 2024;8:2.


