
Histopathological features of giant mediastinal tumors—a literature review
Introduction
The mediastinum can harbor a broad range of neoplasms including thymic epithelial tumors (TET), lymphoproliferative diseases, germ cell tumors (GCTs), mesenchymal tumors, and metastases among others. In addition, benign lesions such as cysts can also occur in that location. The prevalence of solitary lesions in adults varies by the mediastinal compartment (1). In the prevascular mediastinum, the most common solitary lesions in adults are thymomas followed by benign cysts, lymphomas, thymic carcinomas, GCTs, metastases, and others (2). In the visceral mediastinum, benign cysts are followed by metastases, benign thyroid lesions, lymphomas, and small cell carcinomas while in the paravertebral mediastinum, neurogenic tumors represent by far the most common solitary lesions; occasionally benign cysts are identified in the paravertebral compartment (2). The mediastinum encompasses a number of vital organs such as the heart, aorta, superior vena cava (SVC), brachiocephalic vein, esophagus, large airways, and nerves. Giant tumors can impinge on these structures resulting in their compression or obstruction leading to symptoms such as dyspnea, cough, wheezing, chest pain, and SVC syndrome. Giant mediastinal tumors (in this review defined as tumors that are 10 cm and larger) pose, of course, a substantial clinical problem not only because of the symptoms they may cause but also for therapy planning and management of the patient. The correct diagnosis is crucial as some tumors such as TET benefit from surgical treatment if completely resectable while others such as many of the malignant GCTs or lymphomas are treated with chemotherapy and/or radiation.
There are various, partially overlapping, schemes of dividing the mediastinum in compartments, sometimes causing confusion. To standardize their classification and to ease the communication between radiologists, surgeons, pathologists, pulmonologists, medical oncologists, and radiation oncologists, the International Thymic Malignancy Interest Group (ITMIG) developed a multidetector computed tomography (CT)-based definition of mediastinal compartments (3). This clinically-oriented scheme includes three compartments: prevascular, visceral, and paravertebral (Table 1). Importantly, in this model, compartment borders follow true anatomical planes.
Table 1
| Prevascular compartment | Visceral compartment | Paravertebral compartment |
|---|---|---|
| Thymoma, thymic carcinoma | Cysts | Peripheral nerve sheath tumor |
| Germ cell tumor | Lymphoma | Neuroblastic tumor |
| Lymphangioma | Hemangioma | Lymphangioma |
| Angiosarcoma | Liposarcoma | Paraganglioma |
| Synovial sarcoma | Desmoid fibromatosis | Synovial sarcoma |
| Thymolipoma | Cysts | |
| Thymoliposarcoma | Lymphoma | |
| Lipofibroadenoma | Hemangioma | |
| NUT-carcinoma | Liposarcoma | |
| SMARCA4-deficient undifferentiated tumor | Desmoid fibromatosis | |
| Cysts | ||
| Lymphoma | ||
| Hemangioma | ||
| Liposarcoma | ||
| Desmoid fibromatosis |
NUT, nuclear protein in testis.
In this review, we describe, from the pathologist’s point of view, lesions in the mediastinum that most commonly can present as a giant mass. A paragraph of the review will be dedicated to difficulties inherent to small biopsies, where one of the main problems can be the sample’s representativeness. However, it is not feasible to cover all possible giant lesions. Furthermore, benign giant lesions such as cysts can also occur in that location. We present this article in accordance with the Narrative Review reporting checklist (available at https://med.amegroups.com/article/view/10.21037/med-23-23/rc).
Methods
A “giant tumor” was arbitrarily defined as a tumor that measures at least 10 cm in greatest dimension. The 2021 World Health Organization (WHO) classification of mediastinal tumors was searched for tumors reported to be larger than 10 cm (4). Tumors that can present as giant mediastinal lesions based on our own experience were also included. PubMed search was then performed for these lesions (Table S1). Additional manuscripts were selected by reviewing reference lists of relevant publications. Publications, where the diagnosis was not straightforward, were excluded. Additional information on the search strategy is presented in Table 2.
Table 2
| Item | Specification |
|---|---|
| Date of search | February 1st 2023 |
| Databases and other sources searched | 2021 WHO classification of mediastinal tumors was searched for tumors larger than 10 cm. Additionally, tumors larger than 10 cm based on the author’s experience were included. PubMed was then searched for selected entities |
| Search terms used | See Table S1 for details |
| Timeframe | Date unrestricted to February 2023 |
| Inclusion and exclusion criteria | Inclusion: (I) English and German language; (II) case reports, case series, retrospective cohort series, prospective studies; (III) focusing on subtopics of histology and diagnosis |
| Exclusion: same tumors outside of mediastinum, questionable diagnosis | |
| Selection process | L.B. selected literature, both authors chose those to be included |
| Any additional considerations, if applicable | References of selected manuscripts were reviewed for potential inclusion |
WHO, World Health Organization.
We did not include hematolymphoid neoplasms, even though they can present as giant tumors in the mediastinum. However, the large variety of hematolymphoid neoplasms would require a review on its own. Furthermore, for many of these neoplasms biopsies are performed but no resection. If a hematolymphoid neoplasm is in the differential diagnosis of any of the lesions we included, it will be discussed.
Histomorphologic classification of giant mediastinal neoplasms
The histomorphologic classification of giant mediastinal tumors is summarized in Table 3.
Table 3
| Mesenchymal tumors | Tumors of the thymus | Germ cell tumors |
|---|---|---|
| Adipocytic tumors | Epithelial tumors | Teratoma |
| • Thymolipoma | • Thymoma | Mixed germ cell tumors |
| • Liposarcoma | • Thymic carcinoma | Seminoma |
| • Thymoliposarcoma | • NUT carcinoma | Embryonal carcinoma |
| Fibroblastic and myofibroblastic tumors | • Lipofibroadenoma* | Yolk sac tumor |
| • Desmoid fibromatosis | Choriocarcinoma | |
| • Solitary fibrous tumor | ||
| Vascular tumors | ||
| • Hemangioma | ||
| • Lymphangioma | ||
| • Angiosarcoma | ||
| Peripheral nerve sheath and neural tumors | ||
| • Paraganglioma | ||
| • Schwannoma | ||
| • Malignant peripheral nerve sheath tumor | ||
| • Ganglioneuroma | ||
| • Ganglioneuroblastoma | ||
| • Neuroblastoma | ||
| Tumors of uncertain differentiation | ||
| • Synovial sarcoma | ||
| • SMARCA4-deficient undifferentiated tumors |
*, lipofibroadenomas are classified as thymic tumors in the 2021 WHO classification. NUT, nuclear protein in testis; WHO, World Health Organization.
Tumors of blood and lymph vessels
Hemangioma
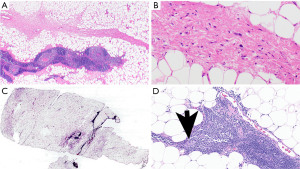
Hemangiomas are benign neoplasms that are classified as mesenchymal tumors of the thorax (4). These tumors are composed of vascular spaces or blood vessels. They can develop throughout the body, and their pathogenesis is unknown. In the mediastinum, they can occur in any compartment (2,5). Mediastinal hemangiomas are rather uncommon, accounting for less than 0.5% to 1% of mediastinal tumors (2,6,7). They are distributed across all age groups and are often asymptomatic. However, since they can be as large as 20 cm, they can cause symptoms due to expansion, including SVC syndrome, various neurological problems, dysphagia, and if ruptured, hemothorax (5). Usually, these lesions are well circumscribed, and only rarely infiltrate neighboring structures. The overall prognosis after surgical treatment is good, even for infiltrative tumors, since they do not progress (6).
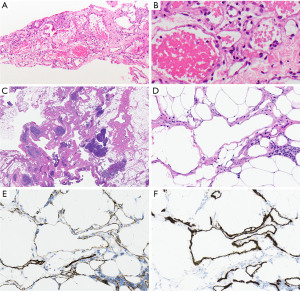
Histologically, hemangiomas are characterized by vascular proliferation, including veins and capillaries (Figure 1A,1B). Even if they show invasive growth, it is mostly limited (8). In between vessels there is fibrous and smooth muscle tissue with inflammatory cells, and occasionally additional changes include myxoid degeneration, hyalinization, and ossification (5). The differential diagnosis includes lymphangioma and angiosarcoma. The distinction of hemangioma from its mimickers is largely based on morphologic characteristics (see below) as the expression patterns of immunohistochemical (IHC) stains can be overlapping.
Lymphangioma
Lymphangiomas are also benign although they can behave more aggressively due to their sometimes infiltrative and expansile growth. They can occur in any mediastinal compartment and encompass 0.3% to 4% of all mediastinal tumors (2,9,10). They are primarily regarded as developmental disorders, and therefore they are more common in children and young adults (11,12). PIK3CA mutations have been identified in lymphangiomas which could be responsible for the aberrant development of lymphatic vessels (13,14). They are mostly cavernous and cystic and measure up to 20 cm in diameter (15). Surgery is the therapy of choice, although sometimes due to localization and infiltrative growth complete resection is impossible (16).
Lymphangiomas have some features that are overlapping with hemangiomas. Lymphangiomas are characterized by the accumulation and proliferation of lymphatic structures usually associated with a lymphocytic infiltrate (Figure 1C,1D). Lymphocytic infiltrates are helpful in the distinction of lymphangiomas from hemangiomas which usually lack that feature. Vessels are empty or contain proteinaceous material possibly with lymphocytes, but without erythrocytes. Endothelial cells are low and flat. Like hemangioma, vessel-lining cells express ERG, CD31 (Figure 1E), and CD34; in addition, they express podoplanin (D2-40) (Figure 1F).
Angiosarcoma
Angiosarcoma is a rare malignant vascular tumor representing less than 1% of all sarcomas (17) and also less than 1% of all mediastinal tumors (2,18-20). They most commonly develop in the prevascular compartment. Patients present with symptoms including dyspnea, cough, hemothorax, and cardiac compression (21,22). They are more common in older men. Interestingly, in children, angiosarcomas most commonly occur in the mediastinum (23). A subset of angiosarcomas develops after radiotherapy; almost all of them harbor MYC amplification (24-27). Others arise in preexisting hemangiomas, after surgery, or trauma. Various angiogenesis-associated genes are mutated in these tumors such as VEGFR2, VEGFR3, TIE1, PLCG1, PTPRB, and KDR (28-31).
Histologically angiosarcomas are characterized by the proliferation of neoplastic epithelioid and/or spindle cells, with vascular structures covered by atypical endothelial cells (Figure 2A-2C). High mitotic rate, necrosis, and hemorrhage are usually present. The neoplastic cells express CD31 (Figure 2D), ERG, Fli-1 (Figure 2E), and CD34 (32-34). Importantly, epithelioid cells can be positive for keratins (Figure 2F) which is a pitfall. Angiosarcoma with MYC amplification will also express MYC.
Features that are helpful in the differential diagnosis of spindle cell neoplasms in the prevascular mediastinum are summarized in Table 4.
Table 4
| Tumor | Morphologic characteristics | Anxillary testing |
|---|---|---|
| Angiosarcoma | • Vascular structures/channels lined by atypical endothelial cells which can be bland to very atypical • Neoplastic spindle and/or epithelioid cells • High mitotic activity, necrosis, and hemorrhage common |
• IHC: vascular markers (CD31, CD34, ERG, Fli-1) Keratin in subset • MYC amplification in radiation-induced sarcoma |
| Schwannoma | • Usually encapsulated • Tumor cells spindled and uniform with elongated nuclei • Tumor cells arranged in Antoni A and B areas • Verocay bodies—hallmark of schwannoma • Hyalinized blood vessels |
• IHC: S100 protein, SOX-10 |
| MPNST | • Dense proliferation of atypical spindle or epithelioid cells with high mitotic activity and necrosis • Cartilage, bone, glandular structures, rhabdomyoblasts (“malignant Triton tumors”) possible |
• IHC: focal S100 protein, SOX-10; subset of tumors shows loss of expression of H3K27me3; epithelioid MPNST: subset shows loss of expression of INI-1, strong S100 protein and SOX-10 expression, intact H3K27me3 |
| Synovial sarcoma | • Monophasic (spindle cells only) or biphasic (epithelial- and spindle-cell components in various proportions) • Epithelial cells can form nests, cords, or glands • Spindle cells: delicate, fairly uniform, relatively small, sparse cytoplasm, ovoid, bland but hyperchromatic nuclei with granular chromatin and inconspicuous nucleoli; growing in dense cellular sheets or vague fascicles • Poorly differentiated areas might be present |
• IHC: SS18-SSX and SSX-C-terminus; epithelial cells: keratin; spindle cells: keratin (focal) in most cases • FISH or RT-PCR (only necessary if fusion-specific IHC not available): t(X;18)(p11;q11) (2/3 of cases SS18-SSX1 fusion, 1/3 SS18-SSX2 fusion, a few cases with SS18-SSX4 fusion) |
| Liposarcoma | • Well-differentiated: adipose tissue with scattered atypical spindle cells with nuclear atypia and dark nuclear chromatin • Dedifferentiated: might harbor well-differentiated liposarcoma areas. Sheets of atypical spindle cells • Myxoid: small, round, stellate cells with variable small lipoblasts in a myxoid stroma • Pleomorphic: bizarre lipoblasts, multinucleated floret-type giant cells |
• Well-differentiated: FISH: MDM2 amplification • Well-differentiated, dedifferentiated: giant ring and marker chromosomes containing amplified sequences of chromosome region 12q13-15 for genes MDM2, CDK4, and CPM • Myxoid: DDIT3 gene rearrangements |
| Desmoid-type fibromatosis | • Poorly circumscribed • Infiltrative • Elongated, slender spindle cells of uniform appearance • No nuclear hyperchromasia or cytologic atypia • Cells grow in long sweeping bundles • Variable mitotic rate • Collagenous stroma with variably prominent blood vessels |
• Usually not necessary • IHC: variable staining for MSA, SMA, nuclear beta-catenin stain (70–75% of tumors) • Molecular: CTNNB1 mutation (85% of tumors) |
| Solitary fibrous tumor | • Usually well-circumscribed • Spindle cells • Ropey collagen • Hemangiopericytoma-like vasculature |
• IHC: STAT6, CD34 • Molecular: NAB2–STAT6 gene fusion (usually not necessary) |
| Micronodular thymoma with lymphoid stroma | • Slightly spindled or oval bland appearing epithelial cells, no or low mitotic activity, forming nodules • Background of B cells forming scattered lymphoid follicles with germinal centers |
• Usually not necessary in resection specimen • IHC: neoplastic cells: keratin AE1/AE3, p40, p63 B cells: CD20 Scattered TdT-positive thymocytes |
| Metaplastic thymoma | • Biphasic • Epithelial component: islands or trabeculae of oval or slightly spindled cells with moderate eosinophilic cytoplasm, very low mitotic activity • Fibroblast-like spindle cell component: Bland spindle cells with pale cytoplasm • Thymocytes absent |
• IHC: epithelial cells: keratin AE1/AE3, p40, p63 Spindle cells: keratin AE1/AE3 +/−; p40/p63− • Molecular: YAP1-MAML2 gene fusion in both components |
IHC, immunohistochemistry; MPNST, malignant peripheral nerve sheath tumor; FISH, fluorescence in situ hybridization; RT-PCR, reverse transcriptase-polymerase chain reaction; MSA, muscle specific actin; SMA, smooth muscle actin.
Tumors of neurogenic origin
In the mediastinum neurogenic tumors arise from neural elements such as the sympathetic system, phrenic, and pneumogastric nerves. The great majority of these tumors (71–95%) occur in the paravertebral mediastinal compartment (2,45). They represent 4% to 34% of all mediastinal lesions; however, in the paravertebral mediastinum they represent 54% of all solitary lesions and are the most common solitary lesion in that compartment (2,7,46). Based on their origin tumors are classified as peripheral nerve sheath tumors [schwannoma, neurofibroma, malignant peripheral nerve sheath tumor (MPNST)], neuroblastic tumors (ganglioneuroma, ganglioneuroblastoma, neuroblastoma), and paraganglia (paraganglioma). While many of the neurogenic tumors in the mediastinum are benign, 5% to 10% and 40% to 60% are considered malignant in adults and children, respectively (47). Patients are mostly asymptomatic. Generally, as a group, patients with neurogenic tumors in the mediastinum have a good prognosis after surgical removal.
Schwannoma
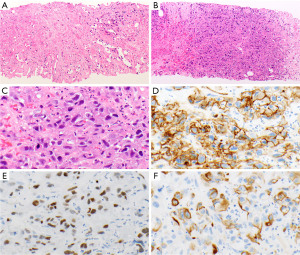
Schwannoma is a benign tumor composed almost exclusively of neoplastic Schwann cells. It is the most common neurogenic tumor of the mediastinum, representing approximately 50% of these tumors occurring at that site (7,46,48,49). They are usually encapsulated (4). Schwannomas are comprised of hypercellular (Antoni A) and hypocellular (Antoni B) areas (Figure 3A). The tumor cells are spindled, mostly uniform, with elongated nuclei (Figure 3B) that diffusely express S100 protein (nuclear and cytoplasmic, Figure 3C) and SOX-10 (35,36). Occasionally hyalinized blood vessels (Figure 3D), nuclear palisading (Verocay bodies, Figure 3E), and chronic inflammation with lymphoid aggregates are present. Up to 75% of schwannomas harbor an inactivating NF2 mutation (50-53). Multiple schwannomas are the hallmark of neurofibromatosis type 2 and schwannomatosis.
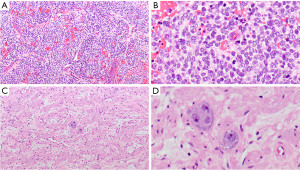
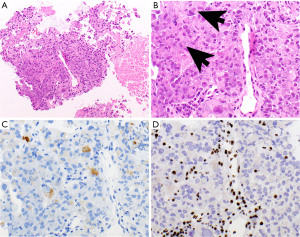
MPNST
MPNSTs are malignant spindle cell tumors, which can grow up to 27 cm. The majority of MPNSTs are associated with neurofibromatosis type 1, followed by sporadic and radiation-induced tumors (37,49). NF1 gene mutations are often found in MPNST (54,55), as well as mutations in CDKN2A/CDKN2B and EED or SUZ12 (56-59). These tumors are characterized by dense populations of spindle cells, high mitotic activity, and necrosis (Figure 4A,4B) (37). MPNSTs may also contain cartilage, bone, glandular structures, and/or rhabdomyoblasts (“malignant Triton tumors”). The neoplastic cells may focally express S100 protein and SOX-10 or are entirely negative for both. H3K27me3 (Figure 4C), if lost, can be helpful in the differential diagnosis (38). Seventy-five percent of epithelioid MPNSTs, a subtype of MPNST harbor an inactivating SMARCB1 (INI1) mutation, demonstrated with loss of INI1 expression by IHC. They are also strongly S100 protein and SOX-10 positive, without loss of H3K27me3 (39). MPNST have a poor prognosis with a mortality of 60% (49,60).
Paraganglioma
Paragangliomas are rare neural crest neoplasms encompassing less than 1% of mediastinal lesions (2). They can occur in any of the mediastinal compartments (2,7,61). In the mediastinum they originate from the sympathetic ganglia. Paragangliomas are usually circumscribed and can sometimes be larger than 10 cm in diameter (62). Histologically they are recognized by epithelioid cells arranged in a nested pattern (“Zellballen”), where epithelioid tumor cells are surrounded by sustentacular cells (Figure 5). Nuclei may sometimes be pleomorphic, with occasionally pronounced nucleoli. Tumor cells have finely granular, eosinophilic, or basophilic cytoplasm. Mitotic activity can be seen in approximately half of paragangliomas (62). The tumor cells express neuroendocrine markers (chromogranin, synaptophysin, INSM1) and are characteristically negative for keratins. Sustentacular cells express S100 protein and GFAP. A subset of mediastinal paraganglioma has been found to harbor single or multiple mutations including SDHB, SDHD, SDHC, ATRX, TERT, and TP53 mutations (62). In most of the patients, germline mutation analysis revealed the same succinate dehydrogenase mutation (or the lack thereof) as identified in the paraganglioma of that patient (62).
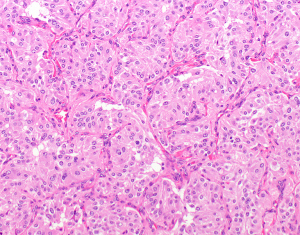
All above mentioned tumors of neurogenic origin may occur over a wide age range, but most commonly in adults.
Neuroblastic tumors
Peripheral neuroblastic tumors (PNTs) of the thorax tend to occur in young patients. They include ganglioneuroma, ganglioneuroblastoma, and neuroblastoma. PNT of the thorax comprise 20% of all PNT, which are the most common neoplasms in the first year of life (4,63,64). Histologically, these neoplasms consist of ganglion cells in different stages of differentiation ranging from neuroblasts to mature ganglion cells. Various amounts of Schwann cells and stroma may also be present. Necrosis, hemorrhage, karyorrhexis, mitosis, calcification, and fibrosis are common. Based on the histologic features the International Neuroblastoma Pathology Committee recognizes four categories of neuroblastic tumors: neuroblastoma (Schwannian stroma-poor, Figure 6A,6B), ganglioneuroblastoma, intermixed (Schwannian stroma-rich), ganglioneuroma (Schwannian stroma-dominant, Figure 6C,6D), and ganglioneuroblastoma, nodular (Schwannian stroma-rich/dominant/poor) (65). The tumor cells are positive for NSE , chromogranin, synaptophysin, and CD56, which can aid in the diagnosis. However, while expressed in the tumor cells of neuroblastic tumors, NSE is not specific for these tumors and indeed is expressed in many other neoplasms, therefore this test is not very useful in the distinction of neuroblastic tumors from its mimickers. Molecular alterations in these tumors include MYC amplification, ALK mutation/amplification, TERT rearrangement, ATRX mutations, and various chromosomal aberrations (66-69). Since therapy is dependent on risk-stratification, and can vary from observation and surgery only (in the low-risk group) to multimodality treatments with surgery, chemotherapy, radiation, and anti-GD2 antibody-based immunotherapy, one universally accepted risk stratification system is needed (70-72). The International Neuroblastoma Risk Group (INRG) classification system takes into account the INRG stage, age, histologic category and grade, MYCN amplification status, and 11q aberration and ploidy (73). However, it is still not accepted as the only risk-stratification system (74).
Mesenchymal neoplasms
Synovial sarcoma
Synovial sarcomas are malignant tumors that are characterized by t(X;18)(p11;q11) resulting in SS18-SSX gene fusion in more than 95% of cases. In the thorax, these tumors most commonly occur in the pleura or lung; only 1.4% of all synovial sarcomas are in the prevascular or paravertebral mediastinum (75-78). The average tumor size in that location is 13.5 cm (range, 6–29 cm). There is a slight male predominance and the mean age of patients is 38 years (range, 14–75 years) (79). Patients are usually symptomatic at the time of presentation and are treated by resection (if possible) and chemotherapy and/or radiation.
Synovial sarcomas are comprised of a monomorphic spindle cell proliferation with (biphasic) or without (monophasic, Figure 7A-7C) an epithelial component. In the mediastinum, most of the synovial sarcomas appear to be monophasic, over half of them exhibit a poorly differentiated morphology with rounded or spindle cells that have higher nuclear grade, rhabdoid cytology, and high mitotic activity (76). Otherwise, these tumors show a hemangiopericytoma-like vasculature, wiry collagen, alternating zones of hyper- and hypocellularity, and mast cells in the stroma (76). In the lung/pleura entrapped pneumocytes may mimic a biphasic synovial sarcoma. Fusion-specific antibodies, SS18-SSX (Figure 7D) and SSX-C-terminus (Figure 7E) have recently been shown, especially in combination, to be highly specific and sensitive for synovial sarcoma (40,41). If not available, cytogenetic and molecular studies may also be used to confirm the diagnosis. The differential diagnosis includes other sarcomas, specifically leiomyosarcoma and MPNST, solitary fibrous tumor, sarcomatoid or biphasic mesothelioma, sarcomatoid carcinoma, melanoma, desmoid-type fibromatosis, and even type A thymomas among others.
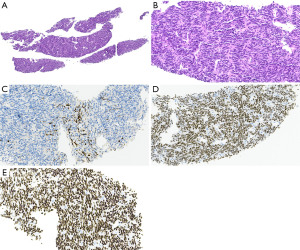
Local recurrence or progressive disease has been reported in almost 90% of patients with mediastinal synovial sarcoma (76). Metastases occur in about half of the patients including to the lung, pericardium, diaphragm, soft tissue, and brain. In a series of 21 mediastinal synovial sarcomas 69% of patients died of the disease 5 to 32 months after diagnosis, the remaining patients were alive with disease (76). Tumor size of over 5 cm and locally recurrent disease have been shown to be independent risk factors for worse outcome (78).
Liposarcoma
Liposarcomas are rare in the mediastinum and comprise less than 1% of all mediastinal lesions (80). They are most commonly found in the paravertebral mediastinum followed by prevascular and visceral mediastinum (80). These tumors can grow up to at least 40 cm (42). The mean age of patients is 53 to 63 years without sex predilection (42,81).
Any of the liposarcoma subtypes can occur in the mediastinum (80). Well-differentiated liposarcomas are the most common subtype in the mediastinum followed by dedifferentiated liposarcoma (80). Pleomorphic liposarcomas appear to be disproportional and more common in the mediastinum than in the soft tissue (42). Myxoid liposarcomas may also occur. Unusual morphologic variants such as lipoleiomyosarcoma, dedifferentiated liposarcoma with “meningothelial”-like dedifferentiation, differentiated myxoid liposarcoma mimicking well-differentiated liposarcoma, and pleomorphic liposarcoma with epithelioid and myxoid change have also been described in this location (42).
Well-differentiated liposarcomas are characterized by adipose tissue with scattered atypical spindle cells with nuclear atypia and dark nuclear chromatin (Figure 8A,8B). Sometimes the distinction from lipoma can be challenging. In these circumstances, MDM2 amplification by fluorescence in situ hybridization (FISH) confirms liposarcoma (43). Dedifferentiated liposarcomas are often arising from well-differentiated liposarcomas and therefore usually harbor well-differentiated areas (Figure 8C). Otherwise, these tumors are comprised of sheets of atypical spindle cells (Figure 8D). Myxoid liposarcomas are composed of small, round, stellate cells with variable small lipoblasts in a myxoid stroma. Pleomorphic liposarcomas are characterized by bizarre lipoblasts (Figure 8E,8F) and possibly multinucleated floret-type giant cells.
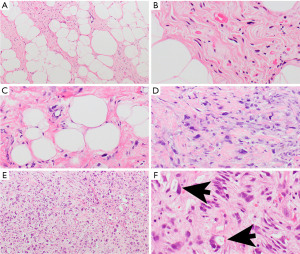
Well-differentiated and dedifferentiated liposarcomas harbor characteristic giant ring and marker chromosomes containing amplified sequences of chromosome region 12q13-15 for genes MDM2, CDK4, and CPM, which can be helpful in the diagnosis (42,43). Myxoid liposarcomas do not carry these amplifications but often harbor DDIT3 gene rearrangements (42).
In the mediastinum, the differential diagnosis of liposarcoma includes lipoma, thymoliposarcoma, thymolipoma, lipofibroadenoma, lipoblastoma (which in general occurs in children less than 3 years old), other sarcomas, and melanoma.
The outcome of liposarcomas is associated with the histologic subtype with worse outcomes of myxoid liposarcomas, followed by pleomorphic liposarcomas (42). Well-differentiated liposarcomas have the best prognosis. Primary mediastinal liposarcomas have a recurrence rate of 10% to 50% after resection; they can metastasize to the lungs, liver, soft tissues, bone, and brain (42).
Thymoliposarcoma
Thymoliposarcomas are extremely rare but are in the differential diagnosis of thymomas, liposarcomas, and thymolipomas. Thymoliposarcomas are usually large tumors ranging from 9 to 35 cm (82,83). Grossly these tumors are described as fatty masses and are usually encapsulated and lobulated (82). On microscopy, lobules of thymic tissue are embedded in the adipose tissue (Figure 9A). The adipose tissue is comprised of benign-appearing and atypical adipocytes, the latter are characterized by hyperchromatic, pleomorphic, and irregular nuclei (Figure 9B). Atypical spindle cells are reported scattered in the adipose tissue and thymic cortex. MDM2 amplification has been reported in one case (83). In a series of 11 cases, the mean age of the patients was 55.8 years (range, 31–77 years) with a slight male predominance (82). Most of the reported cases had a favorable outcome with 4 patients experiencing recurrence and/or metastasis to a vertebra, lymph nodes, and/or lung.
Thymolipoma
Thymolipoma is another rare tumor of the thymus which occurs in the prevascular mediastinum and is classified under mesenchymal neoplasms (adipocytic tumors) in the 2021 WHO classification (4). Thymolipomas have been reported to range in size from 3 cm to more than 30 cm. Since the first description in 1916 (84) less than 300 cases were reported in the English literature. The current name was assigned in 1949 by Hall (85) who described a mixed tumor composed of fat and thymic tissue. Thymolipomas constitute 2% to 9% of thymic tumors and occur over a wide age range (6 months to 82 years), but are more commonly found in younger patients with equal gender distribution (86). In most patients, they will be detected as incidental findings. If symptomatic, patients can present with dyspnea, chronic cough, weight loss, respiratory infections, and even vomiting. Up to 50% of thymolipomas are associated with myasthenia gravis (87,88). They occur very often at the cardiophrenic angle and weigh over 500 g, with a maximal reported weight of 16 kg (88,89). Macroscopically they are usually yellow, soft masses, with a capsule and lobulated appearance. Histologically, most of the tumor (50–95%) is composed of mature adipose tissue (Figure 9C), without any morphologic signs of malignancy. The remainder is comprised of benign, usually involuted thymic parenchyma (Figure 9D) (86,90). A single thymolipoma was described to harbor a t(12,14)(q15q32) (91) suggestive of a dysregulation in the HMGA2 gene which has been identified in the majority of lipomas. If possible, complete surgical resection is curative, without recurrence.
Thoracic SMARCA4-deficient undifferentiated tumor
Thoracic SMARCA4-deficient undifferentiated tumors (SMARCA4-DUT) are very aggressive tumors (92-94). SMARCA4 encodes BRG1, a tumor suppressor, which is a catalytic subunit of the SWI/SNF (BAF) chromatin-remodeling complex. The SWI/SNF complex is involved in the regulation of transcription and the promotion of cell differentiation (94-96). SMARCA4-DUT are characterized by inactivating mutations of SMARCA4 resulting in loss of BRG1 expression in neoplastic cells (97). In general, these tumors also show loss of BRM1 (encoded by SMARCA2, another catalytic subunit of SWI/SNF) although a study of SMARCA4-DUT in the lung showed preserved expression of BRM1 in 18% of the cases (93,98).
The reported median age of these patients ranges between 39 and 59 years (range, 27–82 years) (92,93,95,98,99). There is a male predominance with a male-to-female ratio of up to 9:1. Most patients are smokers. Patients most commonly present with dyspnea, chest pain, and SVC syndrome (92). Tumors are often large at presentation with reported median tumor sizes of 12 to 13 cm (range, 2.2–27 cm). In the thorax, most SMARCA4-DUT are found in the prevascular mediastinum followed by pleura and lung.
These tumors are characterized by poorly differentiated cytology with medium-sized epithelioid cells with irregular nuclear borders and often conspicuous nucleoli (Figure 10A). Sometimes, at least focally, rhabdoid cytology is appreciated (Figure 10B). Focal myxoid stroma or desmoplastic small round cell tumor features are described in 7% of the tumors (99). Mitotic activity is high and extensive necrosis is usually noted. While the tumor cells are forming clusters and sheets, they are growing in a somewhat discohesive pattern. The immunophenotype can vary, however, these tumors show no or only focal keratin expression (Figure 10C) (93,98,99). Loss of expression of BRG1 (Figure 10D) is the hallmark of SMARCA4-DUT. Diffuse expression of SOX2 is reported in almost all cases (94,95,99). About one-third of the cases express CD34 and/or SALL4. Synaptophysin can also be expressed in a subset of cases. Rare tumors focally express claudin 4 and TTF-1. SMARCA4-DUT are negative for desmin, nuclear protein in testis (NUT), S100 protein, WT-1, and p40 (93,98,99). In 69% to 88% of cases a TP53 mutation is identified (93,94).
The pathogenesis of SMARCA4-DUT is not entirely clear. Evidence suggests that these tumors may represent de-differentiated non-small cell carcinomas (93) although some features indicate that they are more closely related to other BAF-deficient tumors such as malignant rhabdoid tumor and small cell carcinoma of the ovary, hypercalcemic type (SCCOHT) (94).
The differential diagnosis of SMARCA4-DUT includes malignant rhabdoid tumor and atypical teratoid/rhabdoid tumor, metastatic SCCOHT, non-small cell lung carcinoma, NUT carcinoma, GCT, lymphoma, malignant melanoma, mesothelioma, and sarcoma (99). Loss of BRG1 expression by itself is not specific for SMARCA4-DUT as it can also be seen in carcinomas including those of lung origin, SCCOHT, and a subset of malignant rhabdoid tumors and atypical teratoid/rhabdoid tumors.
The majority of patients with SMARCA4-DUT has metastases at time of presentation including to lymph nodes (59–91% of patients), adrenal gland (27–48%), lung (29%), and bone (24–55%) (92,93). The median survival of these patients is only 4 to 7 months (range, 1–13 months). Disease progression or relapse occurs in essentially all patients and patients in general die of their disease, mostly due to local complications (92,94). Response to chemotherapy and surgery is limited (92). However, promising preclinical studies and early clinical trials with the H3K27 histone methyltransferase EZH2 inhibitor are underway in tumors related to the SWI/SNF complex emphasizing the importance of an accurate diagnosis of this tumor (94,100). In addition, a few patients with SMARCA4-DUT showed prolonged partial response following treatment with pembrolizumab, an anti-PD-1 antibody (101,102).
Desmoid-type fibromatosis
Desmoid-type fibromatosis is uncommon in the thorax. In a study of 47 such patients, the median age was 45 years (range, 4–96 years) with a female predominance and a median tumor diameter of 6.5 cm (range, 2.2–13 cm) (103). While the lesions were most commonly located in the chest wall (43%), they also occurred in the supraclavicular area, shoulder, axillary area, and mediastinum. Most patients (75%) underwent resection, some in combination with chemotherapy and/or radiation.
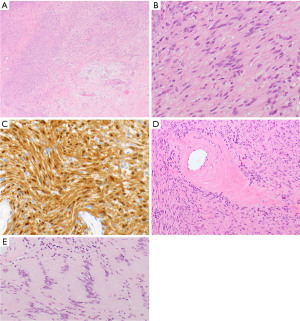
Desmoid-type fibromatosis are poorly circumscribed neoplasms that grow in an infiltrative pattern. The neoplastic spindle cells are bland, elongated, and slender of uniform appearance and are growing in interlacing bundles/long sweeping fascicles in a collagenous stroma with variably prominent blood vessels (Figure 11A,11B). In general, there is no nuclear hyperchromasia or cytologic atypia. The mitotic rate can vary. In 70% to 100% of cases, the tumor cells show nuclear beta-catenin staining due to CTNNB1 mutation that is seen in 85% of these tumors or, in a few cases, mutations in the APC gene (104,105). SMA expression is found in a large subset of fibromatoses (103). The tumor cells are negative for CD34, desmin, and S100 protein (103). The Ki-67 labeling index is low with 1 to 3% of tumor cell nuclei staining (103).
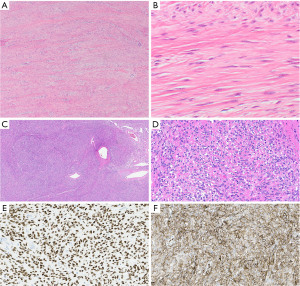
Desmoid-type fibromatosis may recur but does not metastasize; in the study of 47 patients with thoracic fibromatosis 28% of patients had recurrence and 13% progressed (103).
The differential diagnosis of desmoid-type fibromatosis includes solitary fibrous tumor, synovial sarcoma, and desmoplastic mesothelioma.
Solitary fibrous tumor
The solitary fibrous tumor is a spindle cell neoplasm that is usually slow growing, well circumscribed, and only rarely occurs as a primary tumor in the mediastinum. It may be pedunculated. In a study of 70 resected thoracic solitary fibrous tumors only 23% originated from the mediastinum (106). While approximately 30% of all solitary fibrous tumors occur in the thoracic cavity, most of those arise in the pleura (107). Patient’s symptoms are usually due to the mass effect on vital structures. However, patients may also become hypoglycemic and acromegalic due to the production of IGF2 by the tumor (108).
Solitary fibrous tumors are comprised of bland oval and spindled cells growing in a patternless pattern with cells intersected by ropey collagen (Figure 11C,11D). Hemangiopericytoma-like vasculature is characterized by branching or antler-like vessels which are often hyalinized (Figure 11C). Mitotic activity and necrosis are variable. The neoplastic cells express STAT6 (Figure 11E), CD34 (Figure 11F), and Bcl2. Keratins, desmin, SMA, SOX10, and S100 protein are negative. Resected solitary fibrous tumors are stratified based on their risk to metastasize. While various risk stratifications are proposed, the authors use the risk stratification model proposed and refined by Demicco et al. (109) that includes patient age, tumor size, mitotic activity, and the presence or absence of necrosis. Evidence suggests that mediastinal solitary fibrous tumors behave more aggressively than their pleural counterpart although the numbers are small. Studies also have shown that TERT promoter mutations in solitary fibrous tumors are associated with increased TERT mRNA expression and older age, larger tumor size, higher risk classification, and worse event-free survival (110).
The pathogenesis of solitary fibrous tumors appears to be related to a NAB2-STAT6 fusion gene due to paracentric inversion on chromosome 12q13 (111).
In the mediastinum, especially in the prevascular mediastinum, solitary fibrous tumors need to be differentiated from type A thymoma, monophasic synovial sarcoma, localized sarcomatoid mesothelioma, desmoid fibromatosis, and carcinoid tumor.
TET
Thymoma
Thymomas are rare malignant neoplasms that comprise 38% of all solitary prevascular mediastinal lesions in adults (2). They are part of a spectrum of epithelial neoplasms of the thymus. They commonly occur in the 5th to 6th decade of life, although reported age distribution is from 6 to 83 years old, with female predominance. Thymomas comprise up to 85% of TET in adults (112,113).
Thymomas most commonly occur in the prevascular mediastinum. On imaging, they are well-demarcated, usually homogeneous, and round/lobulated. Occasionally they infiltrate surrounding structures. The size of most thymomas is on average less than 10 cm. However, micronodular thymoma with lymphoid stroma (MNTLS) and metaplastic thymoma can reach 15 and 18 cm, respectively. Since only these fulfill our defined size limit of 10 cm, we will discuss them in more detail below.
Patients with thymoma are staged according to the American Joint Committee of Cancer (AJCC)/International Union Against Cancer (UICC) TNM staging system (114), although the Masaoka-Koga system is still often reported as well (115). In many studies, staging and complete resection have been shown to be the most important prognostic factor (113,115-117). Although the overall prognosis is often favorable, thymomas are regarded as malignant due to their invasive and metastatic potential.
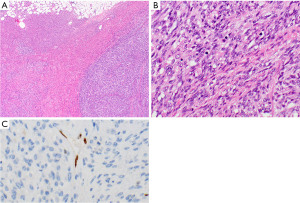
MNTLS occurs in older patients, with a slight male predominance. Patients are usually asymptomatic (118,119). On gross examination, tumors are well demarcated, with a capsule. The cut surface is soft and tan. Morphologically these neoplasms are defined by slightly spindled or oval bland appearing epithelial cells without atypia and no or low mitotic activity comprising small nodules in a background of B cells forming scattered lymphoid follicles with germinal centers (Figure 12A,12B). Scattered TdT-positive thymocytes are usually located at the interface between the epithelial cell nodules and the lymphocytic component. Occasionally cystic changes and rosette-like structures can occur. In up to 30% of MNTLS a component of type A thymoma is also present (119-121). The prognosis is very good, with currently no reported tumor-related deaths, recurrences, or metastases, after surgical removal (119). MNTLS needs to be distinguished from micronodular thymic carcinoma with lymphoid stroma, lymphoepithelial carcinoma, and type AB thymoma.
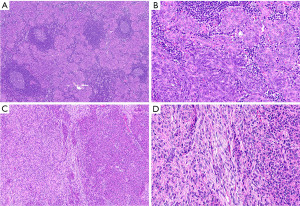
Metaplastic thymoma is a biphasic thymic tumor. It is reported in patients with an age range from 28 to 71 years old and is more common in women. Patients are usually asymptomatic (122-125). Macroscopically these neoplasms are well-demarcated and sometimes have a capsule. The cut surface is grey-white or yellow and solid. Histologically metaplastic thymomas are composed of two more or less well-separated components. The epithelial component comprises islands or trabeculae of oval or slightly spindle cells (Figure 12C,12D). The cytoplasm is moderate and eosinophilic. Mitotic activity is very low. In these areas there is commonly eosinophilic hyaline material present. The other cell component is comprised of fibroblast-like spindle cells with pale cytoplasm. Plasma cells may be present, but thymocytes are usually absent. The majority of metaplastic thymomas does not show invasion and metastases are not reported. After complete surgical resection overall survival is very good (123,126,127). Rare metaplastic thymomas have been reported to transform into sarcomatoid carcinomas (128,129).
YAP1-MAML2 gene fusions have been identified in metaplastic thymoma (44,130).
Lipofibroadenoma of the thymus
Lipofibroadenoma is another rare tumor occurring in the prevascular mediastinum and classified under TET in the 2021 WHO. It is composed of adipose, fibroelastotic, and thymic tissue (Figure 13A-13D). Only case reports of patients with these tumors are available (131-135). Patients are usually asymptomatic, although fever, cough, and night sweats were reported (135). Lipofibroadenomas have been reported up to 23 cm in size (135). Histologically lipofibroadenomas are similar to thymolipomas and fibroadenomas of the breast. It is not clear if this tumor represents a hamartoma or a true neoplasm. It is regarded as a benign tumor, with an excellent prognosis if completely resected.
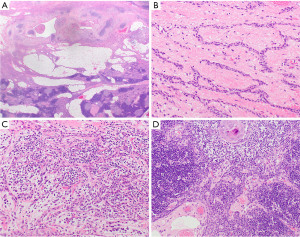
Thymic carcinoma
Thymic carcinomas encompass 7% of all solitary prevascular mediastinal lesions in adults (2). These tumors are usually large and infiltrative and while their mean or median tumor size has been reported between 5.4 and 8.7 cm, they may become as large as 17 cm (136-138). The age range is quite broad with reported median age ranging from 54 to 66 years old (2,136,137,139-143). Thymic carcinoma can be of various subtypes with the most common being squamous cell carcinoma (Figure 14A,14B) (144). Other subtypes include basaloid carcinoma, lymphoepithelial carcinoma, NUT carcinoma (see below), clear cell carcinoma including thymic hyalinizing clear cell carcinoma with EWSR1 translocation, low-grade papillary adenocarcinoma, adenocarcinoma not otherwise specified (NOS), mucoepidermoid carcinoma, thymic carcinoma with adenoid cystic carcinoma-like features, enteric-type adenocarcinoma, adenosquamous carcinoma, sarcomatoid carcinoma, undifferentiated carcinoma, and thymic carcinoma NOS.
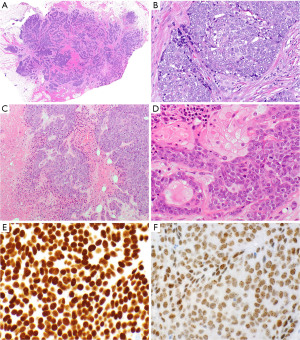
NUT carcinoma
NUT carcinomas are very aggressive tumors that most commonly occur in the midline of the thorax where 51% of all cases are located followed by head and neck (41%) and other sites (145,146). A few NUT carcinomas have been reported outside of the midline. The median age ranges between 16 and 50 years (range, 0.1–80 years). Fifty-one to sixty-seven percent of patients have metastases at the time of presentation. Most commonly a BRD4-NUT fusion due to t(15;19)(q14;p13.1) has been identified although other fusion partners of NUT have also been reported (146-148). This tumor is considered an aggressive subset of squamous cell carcinomas.
Morphologically NUT carcinomas are characterized by a rather monotonous population of epithelioid cells with an increased nuclear-to-cytoplasmic ratio, prominent nucleoli, and open chromatin (Figure 14C,14D). An abrupt squamous differentiation may be noted in a subset of cases (Figure 14D). Specifically in small biopsies there can be crush artifacts leading to a potential misdiagnosis of small cell carcinoma. Large areas of necrosis are usually apparent. The immunoprofile varies; typically, the neoplastic cells are positive for keratin and markers of squamous differentiation such as p40 (Figure 14E) and CK5; they may also express TTF-1 and CD34. NUT immunostain shows a characteristic nuclear stippled expression pattern (Figure 14F) and is 100% specific (after exclusion of a GCT) and 87% sensitive for NUT carcinoma (149). While other assays such as cytogenetics and reverse transcription-polymerase chain reaction (RT-PCR) are available, they are not necessary if the immunostain is positive.
The outcome is usually fatal with a median overall survival of 4.7 to 6.7 months with only rare patients surviving for several years (150,151). The median overall survival is significantly worse in thoracic BRD4-NUTM1 NUT carcinomas (4.4 months) compared with non-thoracic BRD4-NUTM1 NUT carcinomas (10 months) and non-thoracic, non-BRD4-NUTM1 NUT carcinomas (36.5 months) (152). Complete surgical resection and/or initial radiotherapy may be associated with increased progression-free and overall survival (150,153). Clinical trials have been performed or are ongoing using for instance bromodomain and extraterminal domain (BET) family inhibitors to target the BRD4-NUT protein (154,155).
The differential diagnosis includes small cell carcinoma, squamous cell carcinoma, lymphoma, and SMARCA4-DUT.
Primary GCTs of mediastinum
GCTs most commonly occur in gonads. In the mediastinum of adults and children, they comprise up to 5–15% or 20% of neoplasms, respectively (2,156). Although the mediastinum is the most common site of extragonadal GCTs, they only account for 2% to 7% of all GCTs (157,158). Almost all primary mediastinal GCT (PMGCT) develop in the prevascular mediastinum (2,159,160). Evidence suggests that PMGCT arise from primordial germ cells that underwent an aberrant migration during embryogenesis or possibly from stem cells in the thymus (161-164). The incidence of PMGCT abruptly increases with puberty (157,165). While teratomas and yolk sac tumors predominate in prepubertal patients, in postpubertal males a third of the PMGCT are seminomas, a third are teratomas, and a third are mixed GCT, yolk sac tumors, embryonal carcinomas, or choriocarcinomas. In postpubertal females in general only teratomas are found. PMGCT share most of the immunophenotypical and genetic characteristics with gonadal GCT such as the presence of isochromosome 12p or 12p amplification. Unique features include rare PDGFR mutations that only occur in mediastinal, but not in gonadal seminomas (166). Furthermore, PMGCT can be associated with Klinefelter syndrome (167-169). In addition, hematologic malignancies are more commonly associated with PMGCT than GCT elsewhere (170,171). PMGCT often present when they are large at which time the patients experience chest pain, cough, and dyspnea.
Microscopically PMGCT are identical to gonadal counterparts. They are divided into seminomas, non-seminomatous tumors, and teratomas. The most common PMGCT are teratomas, encompassing 43% to 74% of all PMGCTs, followed by seminomas (10–37%) (172,173). Useful immunostains have been presented by Fichtner et al. (174).
Here the most common PMGCT will be shortly presented: teratoma, seminoma, yolk sac tumor, and embryonal carcinoma.
Seminomas occur in postpubertal males usually after the age of 10 years old. Seminomas can grow up to 20 cm in diameter and patients are generally symptomatic. Histologically seminomas are composed of sheets or clusters of uniform, polygonal cells with round nuclei and prominent nucleoli (Figure 15A,15B). The cytoplasm is glycogen-rich, clear to dense eosinophilic. Cell borders are distinct. Usually, there is a background of lymphocytes forming lymphoid follicles with germinal centers (166). Poorly formed non-necrotizing granulomas are often present and can mask the tumor cells leading to a potential wrong assumption of infection (175). In addition, seminomas can present with a lobulated architecture and fibrous bands, features that are similar to thymoma and classic Hodgkin lymphoma, nodular sclerosis type. Lastly, seminomas can be associated with a cyst and therefore the evaluation of the wall of any cyst in the mediastinum is important (176). Indeed, many tumors in the mediastinum can be associated with a cyst including but not limited to seminoma, thymoma, and lymphoma.
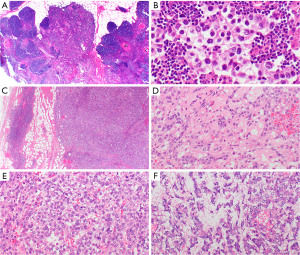
Yolk sac tumors are characterized by various patterns such as glandular-alveolar, solid, hepatoid, macrocystic, microcystic (reticular), endodermal sinus, myxomatous, enteric, polyvesicular-vitelline, and spindle, that often occur together in a single tumor (Figure 15C-15F). Schiller Duval bodies are the hallmarks of yolk sac tumors although they are not always present.
Embryonal carcinoma is an infiltrative malignant tumor growing up to 22 cm, with a poor prognosis. Histologically embryonal carcinoma can also present with various growth patterns, including solid, glandular, and papillary (177), usually with necrotic areas and hemorrhage. Tumor cells are large and polygonal, with clear to eosinophilic and basophilic cytoplasm, prominent nucleoli, and high numbers of mitoses.
Teratomas occur in both sexes and all age groups and can reach up to 25 cm in diameter. While the majority of mediastinal teratomas are mature (Figure 16A,16B), immature teratomas and teratomas with other malignant component(s) such as another GCT, sarcoma, carcinoma, or hematologic malignancy also occur. Pure teratomas derived from nontransformed precursor cells show normal 12p copy numbers and no cytologic atypia and are considered benign (type I GCT). Those teratomas occur in children, females, and some males (178). In postpubertal males, pure teratomas identified in resection specimens post-neoadjuvant chemotherapy in patients who had elevated serum markers including AFP or beta-HCG before neoadjuvant therapy are suggested to derive from malignantly transformed precursor cells, commonly harbor increased 12p copy numbers and show cytologic atypia and are regarded as malignant (type II GCT) (178).
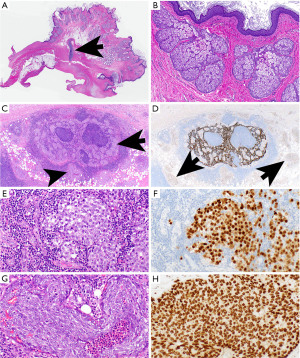
Some PMGCT may be combined with somatic-type malignancies (Figure 16C-16H), or they occur as a combination of two or more PMGCT (mixed GCTs of the mediastinum).
Seminomas have an excellent cure rate with cisplatin-based chemotherapy with 5-year overall survival of 72% to 100%. Five-year overall survival is much worse for patients with non-seminomatous tumors, being only 45% to 60% which is also worse in comparison to similar tumors in other locations (179-181).
Non-neoplastic mediastinal cysts
Mediastinal cysts are rare benign lesions that comprise 12% to 24% of solitary lesions in the mediastinum (2,182). While most mediastinal cysts are identified incidentally, some patients present with symptoms, especially as these cysts can reach as much as 18 cm in diameter (2,183). It is important to differentiate benign cysts from cystic neoplasms or neoplasms that are associated with a mediastinal cyst. Therefore, cystic lesions should be sampled extensively especially if any solid areas or cyst wall irregularities are noted. Based on the pathogenesis mediastinal cysts are classified as derived from embryonic foregut (bronchogenic and enteric cysts), mesothelial derived (pleural and pericardial cysts), thymic cysts, and rare cysts (thoracic duct cyst, mediastinal parathyroid cyst, and cyst with Muellerian differentiation) (182). While the majority of mediastinal cysts are congenital, multilocular thymic cysts, some thoracic duct cysts, and rarely pleuropericardial cysts are acquired (184).
All cysts occur equally in both females and males, except cysts with Muellerian differentiation which occur exclusively in females (184,185).
Bronchogenic cysts are usually unilocular cysts with a smooth surface, and up to 12 cm in diameter (186). They are filled with gelatinous, mucinous, or serous material. The cyst wall is lined by a respiratory type of ciliated pseudostratified epithelium, with or without squamous metaplasia. The cyst wall usually harbors cartilage, seromucinous glands, and/or smooth muscle, and rarely nerves and fat reminiscent of components of a large airway (Figure 17A,17B).
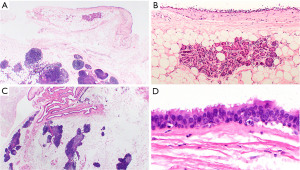
Thymic cysts can be unilocular or multilocular, reaching 18 cm in diameter (187,188). Both unilocular and multilocular cysts have smooth surfaces and contain serous material, however, multilocular cysts can also present with hemorrhagic content. In the cyst wall, there must be thymic tissue. The cyst lining may be comprised of flat, columnar, ciliated, or cuboidal cells, with areas of squamous epithelium (Figure 17C,17D). In multilocular cysts, there are often additional degenerative changes (fibrosis, cholesterol cleft granulomas), inflammation (including lymphoid follicles), and granulation tissue. Since these cysts may be associated with thymic epithelial neoplasms, lymphomas, and PMGCT, thorough sampling is essential (172,187-189).
Enteric cysts (esophageal duplication cyst, gastroenteric and neurenteric cyst) are usually found around the esophagus or in its wall. They can be associated with various congenital anomalies such as tracheoesophageal fistula, esophageal atresia, or congenital pulmonary airway malformation and vertebral malformations (neurenteric cyst) (190,191). Their wall has double-layered smooth muscle, covered with stratified squamous epithelium with or without esophageal glands (esophageal duplication cyst), or gastric/intestinal-type epithelium (gastroenteric cyst).
Mesothelial cysts (celomic, pleuropericardial cysts) also have a smooth surface, usually with a thin wall covered with mesothelial cells. They can also be multilocular and reach a size of up to 18 cm (192).
The therapy of choice for all non-neoplastic mediastinal cysts is complete surgical removal or observation.
Small biopsies
Large lesions in the mediastinum can lead to life-threatening situations due to compression or infiltration of vital structures such as the heart, large airways, lungs, aorta, and SVC. Therefore, the correct diagnosis is important for immediate treatment and management which differs greatly based on the tumor type and possibly clinical stage. Imaging studies together with clinical findings can suffice in some lesions. For instance, in a young male patient with a prevascular mediastinal mass, a high AFP or beta-HCG by serology (>100 IU/L) is virtually diagnostic of a non-seminomatous GCT and a biopsy is not required to start treatment (157,180). Furthermore, a diagnosis of thymoma is often highly suspected based on clinical and imaging features and patients usually undergo resection of that mass without prior biopsy. However, if for instance a lymphoma is suspected, a tissue biopsy to establish the diagnosis is required. Because of the potential heterogeneity of mediastinal lesions, especially given their potentially large size, the results of the biopsy need to be put in context to findings on imaging and clinical impression. For instance, if a biopsy was performed in a young male with a prevascular mediastinal mass and high AFP by serology, however, a biopsy only shows mature teratoma, sampling bias should be suspected as a yolk sac tumor component was probably just not sampled. Similarly, thymomas are in general not subtyped on a biopsy because these tumors can harbor multiple components, and even though a biopsy shows for instance type B2 thymoma, there could be a type A component that was not sampled. Moreover, in thymomas, the initial treatment is in general independent of the subtype and largely relies on the clinical stage of the tumor. However, if there is a suspicion for invasion based on imaging or clinical assessment, and the patient will not undergo primary resection of the tumor, a biopsy should be performed before treatment is initiated. Indeed, with the exception of the above-mentioned young male with non-seminomatous GCT, a biopsy of any tumor that will not be primarily resected but treated with neoadjuvant or adjuvant therapy, should be performed. Biopsies of cysts potentially lack the cyst wall and only show cyst content such as necrotic debris resulting in a “non-diagnostic” biopsy. To minimize sampling bias, multiple needle cores or sampling via mediastinoscopy could be considered. Furthermore, sampling of various areas of the tumor could be helpful to additionally alleviate sampling bias. Because there is increasing potential for targeted therapy for some of the mediastinal lesions requiring testing on paraffin embedded tissue, biopsy specimens should be distributed into multiple tissue cassettes.
Taken together, in large mediastinal lesions, prebiopsy clinical impression and imaging analysis are important to critically evaluate the results of a biopsy and to consider sampling bias. A multidisciplinary discussion is important in the workup of any patient with a mediastinal mass.
Conclusions
A large variety of lesions can present as a giant mass in the mediastinum potentially leading to life-threatening situations given the vital structures in the vicinity. The correct diagnosis of these lesions is important as treatment and management differ substantially from resection of benign cysts for instance to chemotherapy for certain GCTs. In some patients, clinical assessment and imaging are sufficient for a diagnosis, for instance in a young male with a prevascular mediastinal mass and high serologic tumor markers, however, in most patients, a biopsy will be required. Given the potential heterogeneity of lesions, biopsy results must be critically reviewed in the clinical and radiologic context given potential sampling bias.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ryuichi Waseda and Pietro Bertoglio) for the series “Management of Giant Mediastinal Tumors” published in Mediastinum. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://med.amegroups.com/article/view/10.21037/med-23-23/rc
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-23-23/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-23-23/coif). The series “Management of Giant Mediastinal Tumors” was commissioned by the editorial office without any funding or sponsorship. L.B. received grants from Takeda, AstraZeneca, BMS and Roche; he also received payment for lectures and participated in advisory boards from Invitae, Eli-Lilly, AstraZeneca, Roche, MSD, Merck, BMS, Pfizer, Novartis, Takeda, Janssen and received support for attending meeting from Pfizer; he is the International Secretary of the Austrian Society of Pathology/IAP Austria, IASLC Mesothelioma Committee Member and PPS Committee Member. A.C.R. serves as an unpaid Associate Editor of Mediastinum from July 2021 to June 2025; she participated in advisory board of Sanofi and is a consultant to Bristol Meyers Squibb, and received payment or honoraria for Thoracic Course, NY in September 2022, CAP course in September 2022, and Princton Course in April 2023; she received support for attending the WCLC 2022 from IASLC and participating in Sanofi; she is the President-Elect of ITMIG. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Carter BW, Benveniste MF, Madan R, et al. ITMIG Classification of Mediastinal Compartments and Multidisciplinary Approach to Mediastinal Masses. Radiographics 2017;37:413-36. [Crossref] [PubMed]
- Roden AC, Fang W, Shen Y, et al. Distribution of Mediastinal Lesions Across Multi-Institutional, International, Radiology Databases. J Thorac Oncol 2020;15:568-79. [Crossref] [PubMed]
- Carter BW, Tomiyama N, Bhora FY, et al. A modern definition of mediastinal compartments. J Thorac Oncol 2014;9:S97-101. [Crossref] [PubMed]
- WHO Classification of Tumours Editorial Board. Thoracic tumours. 5th ed. Lyon (France): International Agency for Research on Cancer; 2021.
- Moran CA, Suster S. Mediastinal hemangiomas: a study of 18 cases with emphasis on the spectrum of morphological features. Hum Pathol 1995;26:416-21. [Crossref] [PubMed]
- Cohen AJ, Sbaschnig RJ, Hochholzer L, et al. Mediastinal hemangiomas. Ann Thorac Surg 1987;43:656-9. [Crossref] [PubMed]
- Szolkowska M, Szczepulska-Wojcik E, Maksymiuk B, et al. Primary mediastinal neoplasms: a report of 1,005 cases from a single institution. J Thorac Dis 2019;11:2498-511. [Crossref] [PubMed]
- Tan C, Alphonso N, Anderson D, et al. Mediastinal haemangiomas in children. Eur J Cardiothorac Surg 2003;23:1065-7. [Crossref] [PubMed]
- Saleiro S, Magalhães A, Moura CS, et al. Mediastinal cystic lymphangioma. Rev Port Pneumol 2006;12:731-5. [Crossref] [PubMed]
- Brown LR, Reiman HM, Rosenow EC 3rd, et al. Intrathoracic lymphangioma. Mayo Clin Proc 1986;61:882-92. [Crossref] [PubMed]
- Wiegand S, Eivazi B, Barth PJ, et al. Pathogenesis of lymphangiomas. Virchows Arch 2008;453:1-8. [Crossref] [PubMed]
- Alqahtani A, Nguyen LT, Flageole H, et al. 25 years' experience with lymphangiomas in children. J Pediatr Surg 1999;34:1164-8. [Crossref] [PubMed]
- Luks VL, Kamitaki N, Vivero MP, et al. Lymphatic and other vascular malformative/overgrowth disorders are caused by somatic mutations in PIK3CA. J Pediatr 2015;166:1048-54.e1-5.
- Tajima S, Takanashi Y, Koda K. Enlarging cystic lymphangioma of the mediastinum in an adult: is this a neoplastic lesion related to the recently discovered PIK3CA mutation? Int J Clin Exp Pathol 2015;8:5924-8. [PubMed]
- Dionísio AC, Gomes R, Cernadas E, et al. Giant Cystic Mediastinal Lymphangioma. Eur J Case Rep Intern Med 2020;7:001323. [PubMed]
- Knight JK, Marshall MB. Minimally Invasive Management of Complex Recurrent Lymphangioma of the Thorax and Abdomen. Ann Thorac Surg 2016;101:e195-7. [Crossref] [PubMed]
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol 2010;11:983-91. [Crossref] [PubMed]
- Wychulis AR, Payne WS, Clagett OT, et al. Surgical treatment of mediastinal tumors: a 40 year experience. J Thorac Cardiovasc Surg 1971;62:379-92. [Crossref] [PubMed]
- Pachter MR, Lattes R. Mesenchymal tumors of the mediastinum. I. Tumors of fibrous tissue, adipose tissue, smooth muscle, and striated muscle. Cancer 1963;16:74-94. [Crossref] [PubMed]
- Yamazaki K, Minakata K, Nakane T, et al. A rare case of primary angiosarcoma of the anterior mediastinum. Gen Thorac Cardiovasc Surg 2021;69:766-9. [Crossref] [PubMed]
- McCleary AJ. Massive haemothorax secondary to angiosarcoma. Thorax 1994;49:1036-7. [Crossref] [PubMed]
- Miyazaki H, Goto A, Hino R, et al. Pleural cavity angiosarcoma arising in chronic expanding hematoma after pneumonectomy. Hum Pathol 2011;42:1576-9. [Crossref] [PubMed]
- Deyrup AT, Miettinen M, North PE, et al. Angiosarcomas arising in the viscera and soft tissue of children and young adults: a clinicopathologic study of 15 cases. Am J Surg Pathol 2009;33:264-9. [Crossref] [PubMed]
- Killion MJ, Brodovsky HS, Schwarting R. Pericardial angiosarcoma after mediastinal irradiation for seminoma. A case report and a review of the literature. Cancer 1996;78:912-7. [Crossref] [PubMed]
- Lesluyes T, Baud J, Pérot G, et al. Genomic and transcriptomic comparison of post-radiation versus sporadic sarcomas. Mod Pathol 2019;32:1786-94. [Crossref] [PubMed]
- Cornejo KM, Deng A, Wu H, et al. The utility of MYC and FLT4 in the diagnosis and treatment of postradiation atypical vascular lesion and angiosarcoma of the breast. Hum Pathol 2015;46:868-75. [Crossref] [PubMed]
- Mentzel T, Schildhaus HU, Palmedo G, et al. Postradiation cutaneous angiosarcoma after treatment of breast carcinoma is characterized by MYC amplification in contrast to atypical vascular lesions after radiotherapy and control cases: clinicopathological, immunohistochemical and molecular analysis of 66 cases. Mod Pathol 2012;25:75-85. [Crossref] [PubMed]
- Antonescu CR, Yoshida A, Guo T, et al. KDR activating mutations in human angiosarcomas are sensitive to specific kinase inhibitors. Cancer Res 2009;69:7175-9. [Crossref] [PubMed]
- Itakura E, Yamamoto H, Oda Y, et al. Detection and characterization of vascular endothelial growth factors and their receptors in a series of angiosarcomas. J Surg Oncol 2008;97:74-81. [Crossref] [PubMed]
- Italiano A, Chen CL, Thomas R, et al. Alterations of the p53 and PIK3CA/AKT/mTOR pathways in angiosarcomas: a pattern distinct from other sarcomas with complex genomics. Cancer 2012;118:5878-87. [Crossref] [PubMed]
- Behjati S, Tarpey PS, Sheldon H, et al. Recurrent PTPRB and PLCG1 mutations in angiosarcoma. Nat Genet 2014;46:376-9. [Crossref] [PubMed]
- Miettinen M, Lindenmayer AE, Chaubal A. Endothelial cell markers CD31, CD34, and BNH9 antibody to H- and Y-antigens--evaluation of their specificity and sensitivity in the diagnosis of vascular tumors and comparison with von Willebrand factor. Mod Pathol 1994;7:82-90. [PubMed]
- Miettinen M, Wang ZF, Paetau A, et al. ERG transcription factor as an immunohistochemical marker for vascular endothelial tumors and prostatic carcinoma. Am J Surg Pathol 2011;35:432-41. [Crossref] [PubMed]
- Folpe AL, Chand EM, Goldblum JR, et al. Expression of Fli-1, a nuclear transcription factor, distinguishes vascular neoplasms from potential mimics. Am J Surg Pathol 2001;25:1061-6. [Crossref] [PubMed]
- Nonaka D, Chiriboga L, Rubin BP. Sox10: a pan-schwannian and melanocytic marker. Am J Surg Pathol 2008;32:1291-8. [Crossref] [PubMed]
- Karamchandani JR, Nielsen TO, van de Rijn M, et al. Sox10 and S100 in the diagnosis of soft-tissue neoplasms. Appl Immunohistochem Mol Morphol 2012;20:445-50. [Crossref] [PubMed]
- Ducatman BS, Scheithauer BW, Piepgras DG, et al. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer 1986;57:2006-21. [Crossref] [PubMed]
- Schaefer IM, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol 2016;29:4-13. [Crossref] [PubMed]
- Schaefer IM, Dong F, Garcia EP, et al. Recurrent SMARCB1 Inactivation in Epithelioid Malignant Peripheral Nerve Sheath Tumors. Am J Surg Pathol 2019;43:835-43. [Crossref] [PubMed]
- Baranov E, McBride MJ, Bellizzi AM, et al. A Novel SS18-SSX Fusion-specific Antibody for the Diagnosis of Synovial Sarcoma. Am J Surg Pathol 2020;44:922-33. [Crossref] [PubMed]
- Tay TKY, Sukma NB, Lim TH, et al. Correlating SS18-SSX immunohistochemistry (IHC) with SS18 fluorescent in situ hybridization (FISH) in synovial sarcomas: a study of 36 cases. Virchows Arch 2021;479:785-93. [Crossref] [PubMed]
- Boland JM, Colby TV, Folpe AL. Liposarcomas of the mediastinum and thorax: a clinicopathologic and molecular cytogenetic study of 24 cases, emphasizing unusual and diverse histologic features. Am J Surg Pathol 2012;36:1395-403. [Crossref] [PubMed]
- Sciot R. MDM2 Amplified Sarcomas: A Literature Review. Diagnostics (Basel) 2021;11:496. [Crossref] [PubMed]
- Vivero M, Davineni P, Nardi V, et al. Metaplastic thymoma: a distinctive thymic neoplasm characterized by YAP1-MAML2 gene fusions. Mod Pathol 2020;33:560-5. [Crossref] [PubMed]
- Takeda S, Miyoshi S, Minami M, et al. Intrathoracic neurogenic tumors--50 years' experience in a Japanese institution. Eur J Cardiothorac Surg 2004;26:807-12. [Crossref] [PubMed]
- Galetta D, Spaggiari L. Primary Intrathoracic Neurogenic Tumors: Clinical, Pathological, and Long-Term Outcomes. Thorac Cardiovasc Surg 2021;69:749-55. [Crossref] [PubMed]
- Gangadhran SP. Neurogenic tumors of the posterior mediastinum. In: Sugarbaker DJ, Buen R, Krasna MJ, et al. editors. Adult Chest Surgery. 2nd ed. New York: McGraw-Hill; 2009:1145-53.
- Mlika M, Marghli A, Souilem I, et al. A single-institution experience of neurogenic tumors of the mediastinum. Asian Cardiovasc Thorac Ann 2019;27:661-9. [Crossref] [PubMed]
- Boland JM, Colby TV, Folpe AL. Intrathoracic peripheral nerve sheath tumors-a clinicopathological study of 75 cases. Hum Pathol 2015;46:419-25. [Crossref] [PubMed]
- Jacoby LB, MacCollin M, Barone R, et al. Frequency and distribution of NF2 mutations in schwannomas. Genes Chromosomes Cancer 1996;17:45-55. [Crossref] [PubMed]
- Lassaletta L, Torres-Martín M, Peña-Granero C, et al. NF2 genetic alterations in sporadic vestibular schwannomas: clinical implications. Otol Neurotol 2013;34:1355-61. [Crossref] [PubMed]
- Oh JE, Ohta T, Satomi K, et al. Alterations in the NF2/LATS1/LATS2/YAP Pathway in Schwannomas. J Neuropathol Exp Neurol 2015;74:952-9. [Crossref] [PubMed]
- Håvik AL, Bruland O, Myrseth E, et al. Genetic landscape of sporadic vestibular schwannoma. J Neurosurg 2018;128:911-22. [Crossref] [PubMed]
- Mechtersheimer G, Otaño-Joos M, Ohl S, et al. Analysis of chromosomal imbalances in sporadic and NF1-associated peripheral nerve sheath tumors by comparative genomic hybridization. Genes Chromosomes Cancer 1999;25:362-9. [Crossref] [PubMed]
- Bottillo I, Ahlquist T, Brekke H, et al. Germline and somatic NF1 mutations in sporadic and NF1-associated malignant peripheral nerve sheath tumours. J Pathol 2009;217:693-701. [Crossref] [PubMed]
- Lee W, Teckie S, Wiesner T, et al. PRC2 is recurrently inactivated through EED or SUZ12 loss in malignant peripheral nerve sheath tumors. Nat Genet 2014;46:1227-32. [Crossref] [PubMed]
- De Raedt T, Beert E, Pasmant E, et al. PRC2 loss amplifies Ras-driven transcription and confers sensitivity to BRD4-based therapies. Nature 2014;514:247-51. [Crossref] [PubMed]
- Zhang M, Wang Y, Jones S, et al. Somatic mutations of SUZ12 in malignant peripheral nerve sheath tumors. Nat Genet 2014;46:1170-2. [Crossref] [PubMed]
- Pemov A, Hansen NF, Sindiri S, et al. Low mutation burden and frequent loss of CDKN2A/B and SMARCA2, but not PRC2, define premalignant neurofibromatosis type 1-associated atypical neurofibromas. Neuro Oncol 2019;21:981-92. [Crossref] [PubMed]
- Shimizu J, Arano Y, Murata T, et al. A case of intrathoracic giant malignant peripheral nerve sheath tumor in neurofibromatosis type I (von Recklinghausen's disease). Ann Thorac Cardiovasc Surg 2008;14:42-7. [PubMed]
- Muñoz-Largacha JA, Glocker RJ, Moalem J, et al. Incidental posterior mediastinal paraganglioma: The safe approach to management, case report. Int J Surg Case Rep 2017;35:25-8. [Crossref] [PubMed]
- Hsu YR, Torres-Mora J, Kipp BR, et al. Clinicopathological, immunophenotypic and genetic studies of mediastinal paragangliomas†. Eur J Cardiothorac Surg 2019;56:867-75. [Crossref] [PubMed]
- Gurney JG, Ross JA, Wall DA, et al. Infant cancer in the U.S.: histology-specific incidence and trends, 1973 to 1992. J Pediatr Hematol Oncol 1997;19:428-32. [Crossref] [PubMed]
- Gurney JG, Davis S, Severson RK, et al. Trends in cancer incidence among children in the U.S. Cancer 1996;78:532-41. [Crossref] [PubMed]
- Shimada H, Ambros IM, Dehner LP, et al. Terminology and morphologic criteria of neuroblastic tumors: recommendations by the International Neuroblastoma Pathology Committee. Cancer 1999;86:349-63. [Crossref] [PubMed]
- Brodeur GM, Seeger RC, Schwab M, et al. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 1984;224:1121-4. [Crossref] [PubMed]
- Mossé YP. Anaplastic Lymphoma Kinase as a Cancer Target in Pediatric Malignancies. Clin Cancer Res 2016;22:546-52. [Crossref] [PubMed]
- Huang M, Zeki J, Sumarsono N, et al. Epigenetic Targeting of TERT-Associated Gene Expression Signature in Human Neuroblastoma with TERT Overexpression. Cancer Res 2020;80:1024-35. [Crossref] [PubMed]
- Cheung NK, Zhang J, Lu C, et al. Association of age at diagnosis and genetic mutations in patients with neuroblastoma. JAMA 2012;307:1062-71. [Crossref] [PubMed]
- Pinto NR, Applebaum MA, Volchenboum SL, et al. Advances in Risk Classification and Treatment Strategies for Neuroblastoma. J Clin Oncol 2015;33:3008-17. [Crossref] [PubMed]
- Matthay KK, Maris JM, Schleiermacher G, et al. Neuroblastoma. Nat Rev Dis Primers 2016;2:16078. [Crossref] [PubMed]
- Park JR, Bagatell R, London WB, et al. Children's Oncology Group's 2013 blueprint for research: neuroblastoma. Pediatr Blood Cancer 2013;60:985-93. [Crossref] [PubMed]
- Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol 2009;27:289-97. [Crossref] [PubMed]
- Sokol E, Desai AV. The Evolution of Risk Classification for Neuroblastoma. Children (Basel) 2019;6:27. [Crossref] [PubMed]
- Bégueret H, Galateau-Salle F, Guillou L, et al. Primary intrathoracic synovial sarcoma: a clinicopathologic study of 40 t(X;18)-positive cases from the French Sarcoma Group and the Mesopath Group. Am J Surg Pathol 2005;29:339-46. [Crossref] [PubMed]
- Terra SBSP, Aesif SW, Maleszewski JJ, et al. Mediastinal Synovial Sarcoma: Clinicopathologic Analysis of 21 Cases With Molecular Confirmation. Am J Surg Pathol 2018;42:761-6. [Crossref] [PubMed]
- Lan T, Chen H, Xiong B, et al. Primary pleuropulmonary and mediastinal synovial sarcoma: a clinicopathologic and molecular study of 26 genetically confirmed cases in the largest institution of southwest China. Diagn Pathol 2016;11:62. [Crossref] [PubMed]
- Deshmukh R, Mankin HJ, Singer S. Synovial sarcoma: the importance of size and location for survival. Clin Orthop Relat Res 2004;155-61. [Crossref] [PubMed]
- Khalil M, Ahmed MT, Abouelasaad M, et al. A Disastrous Huge Anterior Mediastinal Synovial Sarcoma. Cardiovasc Revasc Med 2022;37:153-5. [Crossref] [PubMed]
- Wong GS, Bass D, Chen IY, et al. Imaging and Clinical Findings in a Series of Six Cases of Rare Primary Mediastinal Liposarcoma. Radiol Cardiothorac Imaging 2022;4:e210259. [Crossref] [PubMed]
- Miura K, Hamanaka K, Matsuoka S, et al. Primary mediastinal dedifferentiated liposarcoma: Five case reports and a review. Thorac Cancer 2018;9:1733-40. [Crossref] [PubMed]
- Alhames S, Ghabally M. Enbloc resection of the largest thymic liposarcoma: A case report with literature review. Ann Med Surg (Lond) 2020;59:204-6. [Crossref] [PubMed]
- Elliott DRF, Myers JL, Konopka KE. Thymoliposarcoma with sebaceous differentiation and MDM2 amplification. Pathol Int 2021;71:633-5. [Crossref] [PubMed]
- Pan CH, Chiang CY, Chen SS. Thymolipoma in patients with myasthenia gravis: report of two cases and review. Acta Neurol Scand 1988;78:16-21. [Crossref] [PubMed]
- Hall GF. A case of thymolipoma with observations on a possible relationship to intrathoracic lipomata. Br J Surg 1949;36:321-4. [Crossref] [PubMed]
- Amălinei C, Grigoraş A, Balan RA, et al. Thymolipoma - the frontier between hamartoma and neoplasia? Rom J Morphol Embryol 2021;62:651-61. [Crossref] [PubMed]
- Hamouri S, Syaj S, Al-Kraimeen L, et al. Thymolipoma and its Association with Myasthenia Gravis: a Multi-center Experience. Med Arch 2021;75:375-81. [Crossref] [PubMed]
- Rieker RJ, Schirmacher P, Schnabel PA, et al. Thymolipoma. A report of nine cases, with emphasis on its association with myasthenia gravis. Surg Today 2010;40:132-6. [Crossref] [PubMed]
- Moran CA, Rosado-de-Christenson M, Suster S. Thymolipoma: clinicopathologic review of 33 cases. Mod Pathol 1995;8:741-4. [PubMed]
- Juanpere S, Cañete N, Ortuño P, et al. A diagnostic approach to the mediastinal masses. Insights Imaging 2013;4:29-52. [Crossref] [PubMed]
- Hudacko R, Aviv H, Langenfeld J, et al. Thymolipoma: clues to pathogenesis revealed by cytogenetics. Ann Diagn Pathol 2009;13:185-8. [Crossref] [PubMed]
- Crombé A, Alberti N, Villard N, et al. Imaging features of SMARCA4-deficient thoracic sarcomas: a multi-centric study of 21 patients. Eur Radiol 2019;29:4730-41. [Crossref] [PubMed]
- Rekhtman N, Montecalvo J, Chang JC, et al. SMARCA4-Deficient Thoracic Sarcomatoid Tumors Represent Primarily Smoking-Related Undifferentiated Carcinomas Rather Than Primary Thoracic Sarcomas. J Thorac Oncol 2020;15:231-47. [Crossref] [PubMed]
- Le Loarer F, Watson S, Pierron G, et al. SMARCA4 inactivation defines a group of undifferentiated thoracic malignancies transcriptionally related to BAF-deficient sarcomas. Nat Genet 2015;47:1200-5. [Crossref] [PubMed]
- Yoshida A, Kobayashi E, Kubo T, et al. Clinicopathological and molecular characterization of SMARCA4-deficient thoracic sarcomas with comparison to potentially related entities. Mod Pathol 2017;30:797-809. [Crossref] [PubMed]
- Schick S, Rendeiro AF, Runggatscher K, et al. Systematic characterization of BAF mutations provides insights into intracomplex synthetic lethalities in human cancers. Nat Genet 2019;51:1399-410. [Crossref] [PubMed]
- Schaefer IM, Cote GM, Hornick JL. Contemporary Sarcoma Diagnosis, Genetics, and Genomics. J Clin Oncol 2018;36:101-10. [Crossref] [PubMed]
- Sauter JL, Graham RP, Larsen BT, et al. SMARCA4-deficient thoracic sarcoma: a distinctive clinicopathological entity with undifferentiated rhabdoid morphology and aggressive behavior. Mod Pathol 2017;30:1422-32. [Crossref] [PubMed]
- Perret R, Chalabreysse L, Watson S, et al. SMARCA4-deficient Thoracic Sarcomas: Clinicopathologic Study of 30 Cases With an Emphasis on Their Nosology and Differential Diagnoses. Am J Surg Pathol 2019;43:455-65. [Crossref] [PubMed]
- Chan-Penebre E, Armstrong K, Drew A, et al. Selective Killing of SMARCA2- and SMARCA4-deficient Small Cell Carcinoma of the Ovary, Hypercalcemic Type Cells by Inhibition of EZH2: In Vitro and In Vivo Preclinical Models. Mol Cancer Ther 2017;16:850-60. [Crossref] [PubMed]
- Henon C, Blay JY, Massard C, et al. Long lasting major response to pembrolizumab in a thoracic malignant rhabdoid-like SMARCA4-deficient tumor. Ann Oncol 2019;30:1401-3. [Crossref] [PubMed]
- Takada K, Sugita S, Murase K, et al. Exceptionally rapid response to pembrolizumab in a SMARCA4-deficient thoracic sarcoma overexpressing PD-L1: A case report. Thorac Cancer 2019;10:2312-5. [Crossref] [PubMed]
- Xu H, Koo HJ, Lim S, et al. Desmoid-Type Fibromatosis of the Thorax: CT, MRI, and FDG PET Characteristics in a Large Series From a Tertiary Referral Center. Medicine (Baltimore) 2015;94:e1547. [Crossref] [PubMed]
- Salas S, Chibon F, Noguchi T, et al. Molecular characterization by array comparative genomic hybridization and DNA sequencing of 194 desmoid tumors. Genes Chromosomes Cancer 2010;49:560-8. [Crossref] [PubMed]
- Garcia-Ortega DY, Martín-Tellez KS, Cuellar-Hubbe M, et al. Desmoid-Type Fibromatosis. Cancers (Basel) 2020;12:1851. [Crossref] [PubMed]
- Zhou C, Li W, Shao J, et al. Thoracic solitary fibrous tumors: an analysis of 70 patients who underwent surgical resection in a single institution. J Cancer Res Clin Oncol 2020;146:1245-52. [Crossref] [PubMed]
- Ronchi A, Cozzolino I, Zito Marino F, et al. Extrapleural solitary fibrous tumor: A distinct entity from pleural solitary fibrous tumor. An update on clinical, molecular and diagnostic features. Ann Diagn Pathol 2018;34:142-50. [Crossref] [PubMed]
- De Los Santos-Aguilar RG, Chávez-Villa M, Contreras AG, et al. Successful Multimodal Treatment of an IGF2-Producing Solitary Fibrous Tumor With Acromegaloid Changes and Hypoglycemia. J Endocr Soc 2019;3:537-43. [Crossref] [PubMed]
- Demicco EG, Wagner MJ, Maki RG, et al. Risk assessment in solitary fibrous tumors: validation and refinement of a risk stratification model. Mod Pathol 2017;30:1433-42. [Crossref] [PubMed]
- Bahrami A, Lee S, Schaefer IM, et al. TERT promoter mutations and prognosis in solitary fibrous tumor. Mod Pathol 2016;29:1511-22. [Crossref] [PubMed]
- Chmielecki J, Crago AM, Rosenberg M, et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet 2013;45:131-2. [Crossref] [PubMed]
- Hsu CH, Chan JK, Yin CH, et al. Trends in the incidence of thymoma, thymic carcinoma, and thymic neuroendocrine tumor in the United States. PLoS One 2019;14:e0227197. [Crossref] [PubMed]
- Weis CA, Yao X, Deng Y, et al. The impact of thymoma histotype on prognosis in a worldwide database. J Thorac Oncol 2015;10:367-72. [Crossref] [PubMed]
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S65-72. [Crossref] [PubMed]
- Detterbeck FC, Nicholson AG, Kondo K, et al. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. J Thorac Oncol 2011;6:S1710-6. [Crossref] [PubMed]
- Roden AC, Yi ES, Jenkins SM, et al. Modified Masaoka stage and size are independent prognostic predictors in thymoma and modified Masaoka stage is superior to histopathologic classifications. J Thorac Oncol 2015;10:691-700. [Crossref] [PubMed]
- Jackson MW, Palma DA, Camidge DR, et al. The Impact of Postoperative Radiotherapy for Thymoma and Thymic Carcinoma. J Thorac Oncol 2017;12:734-44. [Crossref] [PubMed]
- Suster S, Moran CA. Micronodular thymoma with lymphoid B-cell hyperplasia: clinicopathologic and immunohistochemical study of eighteen cases of a distinctive morphologic variant of thymic epithelial neoplasm. Am J Surg Pathol 1999;23:955-62. [Crossref] [PubMed]
- Mneimneh WS, Gökmen-Polar Y, Kesler KA, et al. Micronodular thymic neoplasms: case series and literature review with emphasis on the spectrum of differentiation. Mod Pathol 2015;28:1415-27. [Crossref] [PubMed]
- Qu L, Xiong Y, Yao Q, et al. Micronodular thymoma with lymphoid stroma: Two cases, one in a multilocular thymic cyst, and literature review. Thorac Cancer 2017;8:734-40. [Crossref] [PubMed]
- Tateyama H, Saito Y, Fujii Y, et al. The spectrum of micronodular thymic epithelial tumours with lymphoid B-cell hyperplasia. Histopathology 2001;38:519-27. [Crossref] [PubMed]
- Suster S, Moran CA, Chan JK. Thymoma with pseudosarcomatous stroma: report of an unusual histologic variant of thymic epithelial neoplasm that may simulate carcinosarcoma. Am J Surg Pathol 1997;21:1316-23. [Crossref] [PubMed]
- Yoneda S, Marx A, Müller-Hermelink HK. Low-grade metaplastic carcinomas of the thymus: biphasic thymic epithelial tumors with mesenchymal metaplasia--an update. Pathol Res Pract 1999;195:555-63. [Crossref] [PubMed]
- Chen G, Marx A, Chen WH, et al. New WHO histologic classification predicts prognosis of thymic epithelial tumors: a clinicopathologic study of 200 thymoma cases from China. Cancer 2002;95:420-9. [Crossref] [PubMed]
- Kang G, Yoon N, Han J, et al. Metaplastic thymoma: report of 4 cases. Korean J Pathol 2012;46:92-5. [Crossref] [PubMed]
- Noh TW, Kim SH, Lim BJ, et al. Thymoma with pseudosarcomatous stroma. Yonsei Med J 2001;42:571-5. [Crossref] [PubMed]
- Liu B, Rao Q, Zhu Y, et al. Metaplastic thymoma of the mediastinum. A clinicopathologic, immunohistochemical, and genetic analysis. Am J Clin Pathol 2012;137:261-9. [Crossref] [PubMed]
- Moritani S, Ichihara S, Mukai K, et al. Sarcomatoid carcinoma of the thymus arising in metaplastic thymoma. Histopathology 2008;52:409-11. [Crossref] [PubMed]
- Lu HS, Gan MF, Zhou T, et al. Sarcomatoid thymic carcinoma arising in metaplastic thymoma: a case report. Int J Surg Pathol 2011;19:677-80. [Crossref] [PubMed]
- Zhao J, Zhao R, Xiang C, et al. YAP1-MAML2 Fusion as a Diagnostic Biomarker for Metaplastic Thymoma. Front Oncol 2021;11:692283. [Crossref] [PubMed]
- Moran CA, Zeren H, Koss MN. Thymofibrolipoma. A histologic variant of thymolipoma. Arch Pathol Lab Med 1994;118:281-2. [PubMed]
- Kuo T, Shih LY. Histologic types of thymoma associated with pure red cell aplasia: a study of five cases including a composite tumor of organoid thymoma associated with an unusual lipofibroadenoma. Int J Surg Pathol 2001;9:29-35. [Crossref] [PubMed]
- Qu G, Yu G, Zhang Q, et al. Lipofibroadenoma of the thymus: a case report. Diagn Pathol 2013;8:117. [Crossref] [PubMed]
- Aydin Y, Sipal S, Celik M, et al. A rare thymoma type presenting as a giant intrathoracic tumor: lipofibroadenoma. Eurasian J Med 2012;44:176-8. [Crossref] [PubMed]
- Makdisi G, Roden AC, Shen KR. Successful Resection of Giant Mediastinal Lipofibroadenoma of the Thymus by Video-Assisted Thoracoscopic Surgery. Ann Thorac Surg 2015;100:698-700. [Crossref] [PubMed]
- Roden AC, Yi ES, Cassivi SD, et al. Clinicopathological features of thymic carcinomas and the impact of histopathological agreement on prognostical studies. Eur J Cardiothorac Surg 2013;43:1131-9. [Crossref] [PubMed]
- Hishida T, Nomura S, Yano M, et al. Long-term outcome and prognostic factors of surgically treated thymic carcinoma: results of 306 cases from a Japanese Nationwide Database Study. Eur J Cardiothorac Surg 2016;49:835-41. [Crossref] [PubMed]
- Brown JG, Familiari U, Papotti M, et al. Thymic basaloid carcinoma: a clinicopathologic study of 12 cases, with a general discussion of basaloid carcinoma and its relationship with adenoid cystic carcinoma. Am J Surg Pathol 2009;33:1113-24. [Crossref] [PubMed]
- Sakane T, Murase T, Okuda K, et al. A mutation analysis of the EGFR pathway genes, RAS, EGFR, PIK3CA, AKT1 and BRAF, and TP53 gene in thymic carcinoma and thymoma type A/B3. Histopathology 2019;75:755-66. [Crossref] [PubMed]
- Chalabreysse L, Etienne-Mastroianni B, Adeleine P, et al. Thymic carcinoma: a clinicopathological and immunohistological study of 19 cases. Histopathology 2004;44:367-74. [Crossref] [PubMed]
- Wu J, Wang Z, Jing C, et al. The incidence and prognosis of thymic squamous cell carcinoma: A Surveillance, Epidemiology, and End Results Program population-based study. Medicine (Baltimore) 2021;100:e25331. [Crossref] [PubMed]
- Bakhos CT, Salami AC, Kaiser LR, et al. Thymic Neuroendocrine Tumors and Thymic Carcinoma: Demographics, Treatment, and Survival. Innovations (Phila) 2020;15:468-74. [Crossref] [PubMed]
- Koizumi T, Otsuki K, Tanaka Y, et al. National incidence and initial therapy for thymic carcinoma in Japan: based on analysis of hospital-based cancer registry data, 2009-2015. Jpn J Clin Oncol 2020;50:434-9. [Crossref] [PubMed]
- Thoracic Tumours 5ed. Lyon, France: International Agency for Research on Cancer; 2021.
- Kubonishi I, Takehara N, Iwata J, et al. Novel t(15;19)(q15;p13) chromosome abnormality in a thymic carcinoma. Cancer Res 1991;51:3327-8. [PubMed]
- Suzuki S, Kurabe N, Ohnishi I, et al. NSD3-NUT-expressing midline carcinoma of the lung: first characterization of primary cancer tissue. Pathol Res Pract 2015;211:404-8. [Crossref] [PubMed]
- French C. NUT midline carcinoma. Nat Rev Cancer 2014;14:149-50. [Crossref] [PubMed]
- French CA, Rahman S, Walsh EM, et al. NSD3-NUT fusion oncoprotein in NUT midline carcinoma: implications for a novel oncogenic mechanism. Cancer Discov 2014;4:928-41. [Crossref] [PubMed]
- Haack H, Johnson LA, Fry CJ, et al. Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol 2009;33:984-91. [Crossref] [PubMed]
- Bauer DE, Mitchell CM, Strait KM, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res 2012;18:5773-9. [Crossref] [PubMed]
- Lemelle L, Pierron G, Fréneaux P, et al. NUT carcinoma in children and adults: A multicenter retrospective study. Pediatr Blood Cancer 2017;64: [Crossref] [PubMed]
- Chau NG, Ma C, Danga K, et al. An Anatomical Site and Genetic-Based Prognostic Model for Patients With Nuclear Protein in Testis (NUT) Midline Carcinoma: Analysis of 124 Patients. JNCI Cancer Spectr 2020;4:pkz094. [Crossref] [PubMed]
- Chau NG, Hurwitz S, Mitchell CM, et al. Intensive treatment and survival outcomes in NUT midline carcinoma of the head and neck. Cancer 2016;122:3632-40. [Crossref] [PubMed]
- Schwartz BE, Hofer MD, Lemieux ME, et al. Differentiation of NUT midline carcinoma by epigenomic reprogramming. Cancer Res 2011;71:2686-96. [Crossref] [PubMed]
- Filippakopoulos P, Qi J, Picaud S, et al. Selective inhibition of BET bromodomains. Nature 2010;468:1067-73. [Crossref] [PubMed]
- Grosfeld JL, Skinner MA, Rescorla FJ, et al. Mediastinal tumors in children: experience with 196 cases. Ann Surg Oncol 1994;1:121-7. [Crossref] [PubMed]
- El-Zaatari ZM, Ro JY. Mediastinal Germ Cell Tumors: A Review and Update on Pathologic, Clinical, and Molecular Features. Adv Anat Pathol 2021;28:335-50. [Crossref] [PubMed]
- Rusner C, Trabert B, Katalinic A, et al. Incidence patterns and trends of malignant gonadal and extragonadal germ cell tumors in Germany, 1998-2008. Cancer Epidemiol 2013;37:370-3. [Crossref] [PubMed]
- Sharma P, Jha V, Kumar N, et al. Clinicopathological Analysis of Mediastinal Masses: A Mixed Bag of Non-Neoplastic and Neoplastic Etiologies. Turk Patoloji Derg 2017;33:37-46. [PubMed]
- Moeller KH, Rosado-de-Christenson ML, Templeton PA. Mediastinal mature teratoma: imaging features. AJR Am J Roentgenol 1997;169:985-90. [Crossref] [PubMed]
- Oosterhuis JW, Looijenga LH. Mediastinal germ cell tumors: many questions and perhaps an answer. J Clin Invest 2020;130:6238-41. [Crossref] [PubMed]
- Isaacs H Jr. Perinatal (fetal and neonatal) germ cell tumors. J Pediatr Surg 2004;39:1003-13. [Crossref] [PubMed]
- Frazier AL, Weldon C, Amatruda J. Fetal and neonatal germ cell tumors. Semin Fetal Neonatal Med 2012;17:222-30. [Crossref] [PubMed]
- Oosterhuis JW, Stoop H, Honecker F, et al. Why human extragonadal germ cell tumours occur in the midline of the body: old concepts, new perspectives. Int J Androl 2007;30:256-63; discussion 263-4. [Crossref] [PubMed]
- Stang A, Trabert B, Wentzensen N, et al. Gonadal and extragonadal germ cell tumours in the United States, 1973-2007. Int J Androl 2012;35:616-25. [Crossref] [PubMed]
- Moran CA, Suster S, Przygodzki RM, et al. Primary germ cell tumors of the mediastinum: II. Mediastinal seminomas--a clinicopathologic and immunohistochemical study of 120 cases. Cancer 1997;80:691-8. [Crossref] [PubMed]
- Sudour-Bonnange H, Faure-Conter C, Martelli H, et al. Primary mediastinal and retroperitoneal malignant germ cell tumors in children and adolescents: Results of the TGM95 trial, a study of the French Society of Pediatric Oncology (Societe Francaise des Cancers de l'Enfant). Pediatr Blood Cancer. 2017;64: [Crossref] [PubMed]
- Hasle H, Jacobsen BB, Asschenfeldt P, et al. Mediastinal germ cell tumour associated with Klinefelter syndrome. A report of case and review of the literature. Eur J Pediatr 1992;151:735-9. [Crossref] [PubMed]
- Williams LA, Pankratz N, Lane J, et al. Klinefelter syndrome in males with germ cell tumors: A report from the Children's Oncology Group. Cancer 2018;124:3900-8. [Crossref] [PubMed]
- Nichols CR, Heerema NA, Palmer C, et al. Klinefelter's syndrome associated with mediastinal germ cell neoplasms. J Clin Oncol 1987;5:1290-4. [Crossref] [PubMed]
- Nichols CR, Roth BJ, Heerema N, et al. Hematologic neoplasia associated with primary mediastinal germ-cell tumors. N Engl J Med 1990;322:1425-9. [Crossref] [PubMed]
- Moran CA, Suster S. Primary germ cell tumors of the mediastinum: I. Analysis of 322 cases with special emphasis on teratomatous lesions and a proposal for histopathologic classification and clinical staging. Cancer 1997;80:681-90. [Crossref] [PubMed]
- Takeda S, Miyoshi S, Ohta M, et al. Primary germ cell tumors in the mediastinum: a 50-year experience at a single Japanese institution. Cancer 2003;97:367-76. [Crossref] [PubMed]
- Fichtner A, Richter A, Filmar S, et al. Primary mediastinal germ cell tumours: an immunohistochemical and molecular diagnostic approach. Histopathology 2022;80:381-96. [Crossref] [PubMed]
- Sung MT, Maclennan GT, Lopez-Beltran A, et al. Primary mediastinal seminoma: a comprehensive assessment integrated with histology, immunohistochemistry, and fluorescence in situ hybridization for chromosome 12p abnormalities in 23 cases. Am J Surg Pathol 2008;32:146-55. [Crossref] [PubMed]
- Moran CA, Suster S. Mediastinal seminomas with prominent cystic changes. A clinicopathologic study of 10 cases. Am J Surg Pathol 1995;19:1047-53. [Crossref] [PubMed]
- Moran CA, Suster S, Koss MN. Primary germ cell tumors of the mediastinum: III. Yolk sac tumor, embryonal carcinoma, choriocarcinoma, and combined nonteratomatous germ cell tumors of the mediastinum--a clinicopathologic and immunohistochemical study of 64 cases. Cancer 1997;80:699-707. [Crossref] [PubMed]
- Kao CS, Bangs CD, Aldrete G, et al. A Clinicopathologic and Molecular Analysis of 34 Mediastinal Germ Cell Tumors Suggesting Different Modes of Teratoma Development. Am J Surg Pathol 2018;42:1662-73. [Crossref] [PubMed]
- Kang J, Mashaal H, Anjum F. Mediastinal Germ Cell Tumors. StatPearls. Treasure Island (FL) 2023.
- Stram AR, Kesler KA. Mediastinal Germ Cell Tumors: Updates in Diagnosis and Management. Surg Oncol Clin N Am 2020;29:571-9. [Crossref] [PubMed]
- Kesler KA, Stram AR, Timsina LR, et al. Outcomes following surgery for primary mediastinal nonseminomatous germ cell tumors in the cisplatin era. J Thorac Cardiovasc Surg 2021;161:1947-1959.e1. [Crossref] [PubMed]
- Syred K, Weissferdt A. Non-Neoplastic Mediastinal Cysts. Adv Anat Pathol 2020;27:294-302. [Crossref] [PubMed]
- Bolton JW, Shahian DM. Asymptomatic bronchogenic cysts: what is the best management? Ann Thorac Surg 1992;53:1134-7. [Crossref] [PubMed]
- Strollo DC, Rosado-de-Christenson ML, Jett JR. Primary mediastinal tumors: part II. Tumors of the middle and posterior mediastinum. Chest 1997;112:1344-57. [Crossref] [PubMed]
- Thomas-de-Montpreville V, Dulmet E. Cysts of the posterior mediastinum showing mullerian differentiation (Hattori's cysts). Ann Diagn Pathol 2007;11:417-20. [Crossref] [PubMed]
- Limaïem F, Ayadi-Kaddour A, Djilani H, et al. Pulmonary and mediastinal bronchogenic cysts: a clinicopathologic study of 33 cases. Lung 2008;186:55-61. [Crossref] [PubMed]
- Suster S, Rosai J. Multilocular thymic cyst: an acquired reactive process. Study of 18 cases. Am J Surg Pathol 1991;15:388-98. [Crossref] [PubMed]
- Moran CA, Suster S, El-Naggar A, et al. Carcinomas arising in multilocular thymic cysts of the neck: a clinicopathological study of three cases. Histopathology 2004;44:64-8. [Crossref] [PubMed]
- Hattori H. High-grade thymic carcinoma other than basaloid or mucoepidermoid type could be associated with multilocular thymic cyst: report of two cases. Histopathology 2003;43:501-2. [Crossref] [PubMed]
- Snyder CL, Bickler SW, Gittes GK, et al. Esophageal duplication cyst with esophageal web and tracheoesophageal fistula. J Pediatr Surg 1996;31:968-9. [Crossref] [PubMed]
- Alrabeeah A, Gillis DA, Giacomantonio M, et al. Neurenteric cysts--a spectrum. J Pediatr Surg 1988;23:752-4. [Crossref] [PubMed]
- Manabe T, Oka S, Ono K. Unusual giant multilocular mesothelial cyst of mediastinum. Surg Case Rep 2020;6:249. [Crossref] [PubMed]
Cite this article as: Brcic L, Roden AC. Histopathological features of giant mediastinal tumors—a literature review. Mediastinum 2023;7:37.


