
Perioperative management and postoperative outcomes of locally advanced thymic epithelial tumors: a narrative review
Introduction
Thymic epithelial tumors (TETs), predominantly thymomas and thymic carcinomas, are commonly observed in the anterior mediastinum (1). As reported by the International Thymic Malignancy Interest Group, a significant number of patients with TETs are diagnosed at advanced stages (2). As a result, locally advanced TETs are frequently treated in clinical practice. Understanding the treatment strategies for these cases is crucial. In cases wherein R0 resection is feasible, upfront surgery is considered appropriate for locally advanced TETs. However, if R0 resection is anticipated to be challenging, the National Comprehensive Cancer Network (NCCN) guidelines and the European Society of Medical Oncology (ESMO) treatment guidelines recommend a treatment approach involving chemotherapy or chemoradiotherapy followed by surgery (3,4). Surgical intervention after chemotherapy or chemoradiotherapy poses additional challenges, emphasizing the importance of perioperative management. This article discussed the perioperative management and postoperative outcomes in patients with locally advanced TETs. We present this article in accordance with the Narrative Review reporting checklist (available at https://med.amegroups.com/article/view/10.21037/med-23-24/rc).
Methods
Relevant studies published between 2000 and 2022 were identified through PubMed searches using a combination of the following terms: “locally advanced TETs”, “Thymoma”, “Thymic cancer”, “Surgery”, “Induction therapy”, and “Postoperative outcomes”. The reference lists of the relevant articles were reviewed to identify additional studies. Studies that were considered to have relatively low reliability, such as those with fewer than 10 cases, or those not written in English were excluded from the analysis. Data were extracted based on the relevance of the study to the topic. The initial list of eligible studies was developed by one author and reviewed by other team members until a consensus was reached. Further details regarding the methodology used in this study are presented in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | 4/1/2023 to 4/30/2023 |
| Databases and other sources searched | PubMed |
| Search terms used | “Locally advanced TETs”, “Thymoma”, “Thymic cancer”, “Induction therapy”, “Surgery”, “Postoperative outcomes” |
| Timeframe | 2000–2022 |
| Inclusion criteria | Prospective studies, retrospective studies, meta-analyses, case studies |
| Exclusion criteria | Papers which we considered with low reliability and non-English papers |
| Selection process | The search conducted by M Takenaka and the paper selection was made after discussion with the corresponding author K Kuroda |
In light of the introduction of the new Tumor, Node, Metastasis (TNM) classification for staging locally advanced TETs in 2017 (5), this review article adopts the Masaoka classification as a means to establish a comprehensive staging framework. It is crucial to acknowledge that, within the Masaoka classification, locally advanced TETs are classified as stage III. Nevertheless, it should be noted that the examples of stage IVa and stage IVb in the relevant literature are also included in the scope of this review. Moreover, when transposing these stages to the TNM classification, there is a likelihood that T2–T4 (equivalent to stages II and III) as well as certain stage IVa cases would also be subsumed.
Preoperative management
Only a limited number of prior reports address the preoperative management of TETs. The preoperative considerations for TETs encompass the following (6):
- Evaluation for respiratory symptoms, including the possibility of airway stricture.
- Preoperative evaluation by the anesthesiology department.
- Systematic evaluation of the patient, with a particular focus on potential autoimmune complications.
- Assessment of pharmacotherapy for myasthenia gravis (MG) if MG is present.
The anesthetic management of patients with TETs undergoing thymectomy encompasses several risks associated with potential airway obstruction, hypoxia, and cardiovascular instability. These complications can manifest during various stages, including positioning the patient in the supine position, anesthesia induction, extubation, the immediate postoperative period, and persisting for several days after extubation (7,8). Notably, the presence of respiratory symptoms, evident airway constriction observed on computed tomography (CT) scans, and deviations from normal pulmonary function have been utilized to assess the likelihood of perioperative complications (9).
The anesthetic management of MG necessitates a personalized approach that considers the intricacies of the disease, its treatment, and the potential effects of surgery, anesthesia, and associated medications. The primary goal is to prevent the exacerbation of muscle weakness and to uphold respiratory function. Whenever feasible, the usage of neuromuscular blocking drugs and agents impeding neuromuscular transmission should be minimized (10). Regarding the preoperative medical management of MG, the administration of anticholinesterase drugs and glucocorticoids should be sustained throughout the perioperative period, as discontinuation might worsen symptoms. On the other hand, nonsteroidal immunosuppressive agents, with their prolonged action duration, are less likely to be significantly impacted by perioperative discontinuation (10).
History of induction therapy
In general, early-stage tumors with well-defined borders of the TETs are easily resectable. However, locally advanced TETs classified as Masaoka stage III or higher often show invasion of the pericardium, great vessels, and lungs. This makes complete resection challenging, depending on the tumor location and degree of invasion. Consequently, these tumors have a high risk of incomplete resection, leading to a higher likelihood of recurrence (11). Consequently, when surgery is performed as the initial treatment for Stage III TETs, the rates of complete resection vary and are generally not favorable, ranging from 40% to 90% in the reported series (12-14). Therefore, preoperative treatment is administered for locally advanced TETs to increase the rate of complete resection. Recently, it has been reported that the number of invasive structures is a prognostic predictor of cancer-specific survival in patients undergoing surgery for locally advanced thymoma (15). The number of invasive structures is a factor not expressed in the TNM or Masaoka classification and is interesting from the perspective of defining local advance.
Since the efficacy of induction therapy was first reported by Giaccone et al. in the 1980s (16), numerous studies have investigated its use of induction therapy for TETs. However, approaching the existing literature on induction therapy for TETs with caution is important. Many of the available studies were retrospective and heterogeneous, encompassed both thymoma and thymic carcinoma, and often lacked a comparison group of patients who underwent surgery without induction therapy (17). Furthermore, owing to the rarity of this disease, published prospective clinical trials are scarce, with most studies being limited to single-arm phase II studies (18-24).
There is no consensus regarding whether chemotherapy or chemoradiotherapy should be selected as the induction therapy for locally advanced TETs. However, some reports have suggested that the treatment approach should be based on the histology of TETs (25,26). In thymic carcinoma, complete resection is an unequivocally favorable prognostic factor (27-29), and planning the treatment appropriately is crucial to achieve microscopic complete resection. Therefore, the preferred treatment is chemoradiotherapy, which has a high local therapeutic efficacy. Conversely, in thymomas, while complete resection is the primary goal, debulking surgery has shown potential effectiveness (30,31); surgery is typically performed after chemotherapy, and radiation is added postoperatively. A retrospective report from the European Society of Thoracic Surgeons (ESTS) database revealed that 76% of patients with locally advanced thymoma received chemotherapy as induction therapy (32). Furthermore, Wei et al. reported that, in the Chinese Alliance for Research in Thymomas (ChART) database, chemotherapy was chosen more frequently as the preferred induction therapy over chemoradiotherapy after 2004, as compared to before 2003 (33). Regimens commonly used as induction therapy to alleviate locally advanced TETs include ADOC (adriamycin, cisplatin, vincristine, cyclophosphamide), PAC (cisplatin, doxorubicin, cyclophosphamide), VIP (cisplatin, etoposide, ifosfamide), CAMP (cyclophosphamide, cisplatin, doxorubicin and prednisolone or cisplatin, doxorubicin and methylprednisolone), CP (carboplatin, paclitaxel), and CODE (cisplatin, vincristine, doxorubicin, etoposide).
Wang et al. (34) highlighted the advantages of induction therapy, including (I) providing objective clinical benefits by reducing tumor size and alleviating symptoms; (II) downstaging tumors, which can potentially convert initially unresectable tumors into resectable ones; (III) enhancing early local and systemic disease control; and (IV) demonstrating the feasibility and effectiveness of specific drugs and therapies. These benefits underscore the value of induction therapy in the management of TETs. However, one must note that chemotherapy or chemoradiotherapy used as induction therapy can lead to the development of dense fibrosis in structures affected by tumor invasion. Fibrosis can pose a challenge for surgical dissection. Consequently, en bloc resection may be necessary, even in cases wherein no definitive tumor invasion is confirmed histologically (18).
Combined resection of surrounding organs and R0 resection
The primary approach for treating TETs is to achieve complete resection of the tumor and its invasive sites (12,35). The ability to successfully perform thorough surgical resection is a crucial factor that significantly influences postoperative recurrence rates and overall survival (OS) outcomes (12,36). Previous reports of locally advanced TETs since 2000 are shown in Table 2, though some cases included Masaoka stage IVb. Previous studies reported significant variations in the rates of complete resection following induction therapy for locally advanced TETs, ranging from 15% to 95% (18-25,33,34,37-44). This wide range of outcomes was likely due to the heterogeneous nature of locally advanced TETs. In cases of locally advanced stage III TETs, resection can be relatively easier, and complete resection is feasible when the invasion is limited to structures such as the lungs, pericardium, or phrenic nerves. However, when the invasion involves critical structures, such as the main pulmonary artery or aorta, achieving complete resection becomes more challenging. These cases often require extracorporeal circulation during surgery, which can lead to a higher likelihood of unresectability.
Table 2
| Author | Year | Journal | Country | Study design | Induction treatment | Induction therapy chemo regimens | Histology | Eligibility stage criteria | Masaoka stage | Total (n) |
|---|---|---|---|---|---|---|---|---|---|---|
| Venuta (18) | 2003 | Ann Thorac Surg | Italy | Prospective | CT | Cisplatin-based regimens, 3 | Thymoma: 11, thymic carcinoma: 4 | Unresectable III | – | 15 |
| Kim (19) | 2004 | Lung Cancer | USA | Prospective | CT | CAMP (Kim) 3 course | Thymoma | Unresectable III, IVa and IVb | III: 11, IVa: 10, IVb: 1 | 22 |
| Lucchi (20) | 2005 | Ann Thorac Surg | Italy | Prospective | CT | CEE 3 course | Thymoma | III and IVA | III: 25, IV: 11 | 36 |
| Yokoi (37) | 2007 | J Thorac Oncol | Japan | Retrospective | CT | CAMP (Yokoi) | Thymoma | Unresectable III, IVa and IVb | III: 4, IVa: 6, IVb: 4 | 14 |
| Wright (38) | 2008 | Ann Thorac Surg | USA | Retrospective | CRT | PE 2 course + RT 45 Gy | Thymoma | Unresectable III and IVa | III: 7, IVa: 3 | 10 |
| Kunitoh (21) | 2010 | Br J Cancer | Japan | Prospective | CT | CODE | Thymoma | Unresectable III | – | 21 |
| Mineo (39) | 2010 | Ann Surg Oncol | Italy | Retrospective | CT | PE 3 course | Thymoma | Unresectable III | – | 33 |
| Cardillo (40) | 2010 | Eur J Cardiothorac Surg | Italy | Retrospective | CT | CAMP (Kim) 3–4 course | Thymoma, thymic carcinoma | Unresectable III and IVa | III: 18, IVa: 13 | 31 |
| Rea (41) | 2011 | Lung Cancer | Italy | Retrospective | CT | ADOC 3–4 course | Thymoma, thymic carcinoma | Unresectable III, IVa and IVb | III: 23, IVa: 12, IVb 3 | 38 |
| Park (22) | 2013 | J Thorac Oncol | Korea | Prospective | CT | CDDP + TXT, 3 course | Thymoma: 9, thymic carcinoma: 18 | III and IV | III: 8, IVa: 17, IVb: 2 | 27 |
| Korst (23) | 2014 | J Thorac Cardiovascular Surgery | USA | Prospective | CRT | PE 2 course + RT 45 Gy | Thymoma, thymic carcinoma | III, IV and I and II (>5 cm) | I: 2, II: 4, III: 12, IVa: 1, IVb: 2 | 22 |
| Shintani (25) | 2015 | Gen Thorac Cardiovasc Surg | Japan | Retrospective | CRT or CT | CDDP + TXT, CBDCA + PTX, CODE, PE, ADOC + RT (40–60 Gy) (n=12) | Thymic carcinoma | III and IV | III: 11, IVb: 5 | 16 |
| Cardillo (42) | 2016 | Lung Cancer | Italy | Retrospective | CRT or CT | ADOC, PAC, CEE | Thymoma, thymic carcinoma | III | – | 108 |
| Wei (33) | 2016 | J Thorac Dis | China | Retrospective | CRT or CT | Any | Thymoma, thymic carcinoma NET | III and IV | N/A | 68 |
| Kanzaki (43) | 2019 | Interact Cardiovasc Thorac Surg | Japan | Retrospective | CRT or CT | Any | Thymoma | III and IV | III: 12, IVa: 13, IVb: 4 | 29 |
| Fan (24) | 2020 | Int J Radiat Oncol Biol Phys | China | Prospective | CRT | PE + IMRT 60 Gy | Thymoma: 22, thymic carcinoma: 34 | III and IV | III: 8, IVa: 6, IVb: 42 | 56 |
| Yu (44) | 2021 | Thorac Cancer | China | Retrospective | CRT | Platinum based regimens + RT 40–60 Gy | Thymoma | III and IV | III: 10, IVa: 8, IVb: 1 | 19 |
| Wang (34) | 2021 | Front Oncol | China | Retrospective | CRT or RT or CT | any | Thymoma, thymic carcinoma | III and IV | N/A | 81 |
CT, chemotherapy; CAMP (Kim), cyclophosphamide + cisplatin + doxorubicin + prednisolone; CEE, cisplatin + epirubicin + etoposide; CAMP (Yokoi), cisplatin + doxorubicin + prednisolone; CRT, chemoradiotherapy; PE, cisplatin + etoposide; RT, radiotherapy; CODE, cisplatin + vincristine + doxorubicin + etoposide; ADOC, adriamycin + cisplatin + vincristine + cyclophosphamide; CDDP, cisplatin; TXT, docetaxel; CBDCA, carboplatin; PTX, paclitaxel; PAC, cisplatin + doxorubicin + cyclophosphamide; IMRT, intensity modulated radiation therapy; NET, neuroendocrine tumor; N/A, not available.
Kunitoh et al. attributed the incomplete resection of locally advanced TETs to various factors, including invasion of the pulmonary artery trunk and superior vena cava (SVC) and aortic, myocardial, and sternal invasion (21). In contrast, Mineo et al. identified intrathoracic extension of the tumor and significant vascular invasion as contributing factors to incomplete resection (39). These studies highlighted the specific tumor characteristics and anatomical involvement that can hinder complete resection in locally advanced TETs. In most cases, invasion of the SVC can be addressed through resection using an artificial vessel bypass. However, the feasibility of reconstruction depends on the extent of invasion. In some instances, the reconstruction of the SVC can pose challenges due to the extent of the invasion and the complexity of the procedure. In cases wherein the tumor invades the aorta, achieving complete resection is often challenging, and incomplete resection may be the outcome. However, there have been reports wherein combined resection with aortic replacement was performed, which was also reported by Shintani et al. (25). Notably, a case of thymic carcinoma with aortic invasion was successfully treated with complete resection, and the patient has remained recurrence-free for a period of 3 years (45). These instances demonstrated that complete resection with aortic involvement is possible in select cases, leading to favorable outcomes. Several reports exist regarding the complete resection of TETs with aortic or cardiac invasion using cardiopulmonary bypass, yielding relatively favorable outcomes (46-48). The risk of systemic dissemination of tumor cells while employing cardiopulmonary bypass is deemed acceptable and seems to be low when juxtaposed with the risk of hematogenous tumor cell dissemination triggered by vascular invasion of malignant thymoma or thymic carcinoma (49,50).
On the other hand, among TETs, the challenge of achieving radical resection whenever feasible while considering the risk of potential postoperative complications from aggressive surgery has been highlighted in the context of locally advanced thymomas. In a study by Mastromarino et al., it was concluded that even in cases of locally advanced thymomas resulting in incomplete resection, there were promising 5- and 10-year cancer-specific survival rates of 88% and 80%, respectively (51). This suggests that failure to achieve radical surgery does not preclude the possibility of a cure.
Surgical approach
The choice of surgical approach is a crucial factor in achieving complete resection of TETs. The selection of an optimal approach should prioritize adequate surgical visualization. Although median sternotomy is considered the gold standard for thymectomy, its efficacy may be limited in cases where the tumor extends beyond the mediastinum or involves the pulmonary hilum on either side. In such scenarios, alternative approaches are necessary to ensure a clear and comprehensive field of view. For tumors extending cephalad to the mediastinum or towards the cervical or subclavian regions, a transmanubrial approach (TMA) is often employed to gain better access (52). Additionally, for tumors involving the pulmonary hilum, a T-shaped incision or hemiclamshell approach may be required to achieve an optimal visual perspective (53). In particular, the hemiclameshell approach offers a wider field of view and greater exposure. By utilizing these alternative approaches, surgeons can enhance their visualization capabilities, enabling a more thorough evaluation and complete tumor resection in TETs cases with complex anatomical involvement.
Conventional median sternotomy used for the surgical management of early-stage TETs has several alternative approaches. These include:
- Anterior chest wall lifting method (54): this method is predominantly employed within a unilateral video-assisted thoracoscopic surgery (VATS) approach. The procedure entails raising the anterior chest wall to facilitate access to the thymus, presenting an alternative avenue to sternotomy. The technique of anterior chest wall elevation has demonstrated particular efficacy in cases involving resection of cephalic thymic tissue via a thoracoscope. Nevertheless, Suda et al. (55) have noted that a comparable field of view can be attained through the application of carbon dioxide (CO2) insufflation.
- Bilateral VATS (56): this minimally invasive approach uses small incisions and a thoracoscope to visualize and remove the thymus from both sides of the chest. In comparison to the unilateral VATS approach, this method affords sufficient coverage for both left and right thymectomy; however, a notable drawback is the prolonged duration of the operative procedure.
- Subxiphoid single-port thymectomy (57): in this approach, access to the thymus is achieved through a small single incision positioned below the xiphoid process of the sternum, facilitating thymus removal. Given that this single-port thymectomy procedure can be executed via a solitary 3.5-cm incision in the abdominal region, typically concealed beneath clothing, it boasts exceptional aesthetic qualities. Moreover, it ranks among the least invasive thymectomy techniques due to the absence of sternal incision and the avoidance of intercostal nerve injury. Suda et al. (58) documented that in comparison to VATS thymectomy, single-port thymectomy exhibited comparable operative duration, reduced blood loss, and expedited cessation of postoperative analgesic requirements. Nonetheless, this technique is not without its limitations. It necessitates the utilization of specialized instruments endowed with tip flexibility and mandates CO2 insufflation. Additionally, the constrained surgical field accentuates the vulnerability to instrument interference.
- Dual-port thymectomy using subxiphoid approach (59): the dual-port thymectomy, an advancement achieved by incorporating an additional port into the single-port thymectomy setup, significantly streamlines the surgical procedure by obviating the risk of interference between forceps manipulated by the surgeon’s two hands. Furthermore, akin to the subxiphoid single-port thymectomy, insertion of a camera scope through a subxiphoid incision allows for the acquisition of the same operative field as achieved through a median sternotomy with the dual-port technique. Should the complexity of the procedure warrant, the maneuverability can be enhanced by introducing additional ports. Notably, this technique’s chief benefit lies in its capacity to deliver a favorable field of vision and elevated maneuvering ease.
- Robot-assisted thymectomy (55,60): robotic surgical systems are used to perform thymectomy, providing enhanced precision and dexterity to the surgeon. In robot-assisted thymectomy, the approach employed tends to differ across institutions, encompassing the utilization of both the lateral approach and the subxiphoid approach utilizing the dual-port technique. Irrespective of the chosen approach, the procedures are conducted using three to four robotic arms, thereby enabling the execution of precise and high-quality surgery within a three-dimensional field of view.
These examples demonstrate some alternative approaches available for TETs surgery, with each technique offering unique advantages and considerations. In addition to the aforementioned methods, many institutions have implemented the use of CO2 insufflation to enhance the surgical field during TETs surgery (61). CO2 insufflation involves introduction of carbon dioxide gas into the chest cavity, creating a pneumothorax. This technique helps improve visibility by creating a clear working space and reducing bleeding during the procedure.
Recently, sporadic reports have highlighted the effectiveness of minimally invasive approaches in managing locally advanced TETs (62-64). Minimally invasive approaches are expected to play a significant role in the surgical management of locally advanced TETs in the future. Kang et al. reported 110 cases of robotic thymectomy via the subxiphoid approach, including 67 cases of thymoma and 7 cases of Masaoka stage III or higher (65). This includes cases of combined resection of the pericardium, brachiocephalic vein, and lungs with robot-assisted thoracic surgery (RATS), and we expect to see more reports of combined resection of surrounding organs and angioplasty with minimally invasive approaches such as VATS and RATS in the future.
As technological advances and surgical techniques continue to evolve, there is a growing trend towards employing minimally invasive procedures in the treatment of various thoracic diseases, including TETs. However, notably, in cases of advanced thymomas classified as Masaoka stage III or above, conventional median sternotomy is still commonly used due to the complexities associated with the resection of the surrounding organs (23). The rationale behind adopting a median sternotomy as the surgical approach for locally advanced TETs could stem from the surgeon’s conviction that the paramount objective of the procedure is to achieve radicality through the comprehensive excision of the tumor. Additionally, considering the intricacies of the operation, ensuring safety remains a crucial consideration. One must note that the suitability of a minimally invasive approach for locally advanced TETs depends on various factors, including the extent of tumor invasion, involvement of adjacent structures, surgeon expertise, patient-specific considerations, and safety of surgery. Each case should be evaluated individually, and the selection of the surgical approach should be based on a comprehensive assessment of these factors.
Vascular resection and reconstruction
In recent years, numerous reports have highlighted the surgical technique of combined SVC resection for locally advanced TETs (44,66). This approach involves removal of the thymic tumor along with a portion of the SVC if the tumor invades or compresses a major blood vessel. In the past, patients with SVC lesions were often deemed ineligible for surgery because of their high surgical complexity and associated risks. However, with ongoing innovations in the field, management of SVC lesions has improved remarkably. These advancements have led to the expansion of surgical indications and the ability to offer potentially curative procedures to patients who were previously considered unsuitable for surgery (67,68). The 5-year OS rate after radical surgery with SVC resection for advanced-stage TETs ranges from 45% to 58.1%. However, the rate of major complications ranged from 11.1% to 65% (67,69-71). In contrast, a recent retrospective study suggested that the presence or absence of concomitant SVC resection was not a poor prognostic factor for OS or progression-free survival (PFS) (44).
Generally, patchplasty is recommended when tumor invasion into the SVC or brachiocephalic vein is less than 30% of the vessel wall (72). However, when the invasion exceeds 50%, complete resection of the vessel and reconstruction with an artificial vessel are recommended (69). If SVC blockage occurs without well-developed collateral vessels or a bypass, it can result in decreased cerebral venous return, reduced cardiac output, increased cerebral venous pressure, and potential complications, such as hemodynamic compromise, brain damage, and airway edema (73). Therefore, additional measures are often necessary to block the SVC. Many centers utilize a technique involving a bypass of the brachiocephalic vein to the right atrium using an artificial blood vessel along with tumor resection (74).
Artificial vessels with inner diameters of 10–14 mm for the brachiocephalic vein and 12–20 mm for the SVC are commonly used in revascularization. However, the risk of thrombus-related obstruction is a natural concern when using artificial vessel replacements because veins are low-pressure systems. Previous reports demonstrated a wide range of graft patency rates after vein replacement, ranging from 62% to 100% (75). This variability is influenced by several factors, including the material and diameter of the artificial vessel, surgical technique employed, and perioperative anticoagulation therapy.
Figure 1 shows the most common revascularization methods for the SVC and brachiocephalic veins (76). However, there is no consensus regarding the preferred reconstruction method, and different institutions have adopted different approaches. Although some reports suggest that using the largest possible vessel and performing reconstruction with a single artificial vessel is preferable (69,70), bypassing the left brachiocephalic vein has notable challenges, which has been linked to a high occlusion rate (77). Shintani et al. suggested that left brachiocephalic vein grafts are longer, resulting in a relatively slower blood flow velocity and an increased risk of occlusion. Therefore, they recommended avoiding reconstruction of the left brachiocephalic vein alone (78). They further advised separate reconstruction of each bilateral brachiocephalic vein because anastomosis between Y-shaped grafts is prone to thrombus occlusion. In contrast, Sekine et al. emphasized the importance of selecting a relatively thin graft (8–10 mm) with a wide anastomotic opening to maintain sufficient flow velocity and prevent thrombus formation (79). They also highlighted the importance of avoiding graft torsion (79,80) to ensure successful reconstruction. These differing viewpoints indicate the complexities involved in selecting the optimal approach for SVC and brachiocephalic vein reconstruction, emphasizing the need for individualized decision making based on specific patient factors and surgical expertise.
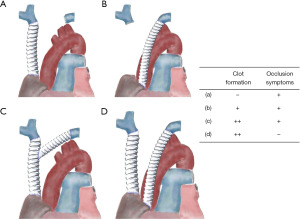
However, the choice of materials for artificial vessel reconstruction remains debatable. Many centers opt for ring-shaped polytetrafluoroethylene (PTFE) grafts because of their favorable characteristics. PTFE grafts offer good patency, and the rigidity of the ring structure helps prevent graft collapse and withstand compression (69). Compared to other synthetic grafts, PTFE has a lower propensity for thrombus formation, allows for immediate postoperative re-epithelialization, and carries a lower risk of infection and platelet deposition. Natural materials, such as the bovine pericardium, also offer advantages over synthetic options, including a reduced risk of infection and decreased likelihood of thrombosis. In particular, tubed bovine pericardial conduits have a lower incidence of infections and thrombosis (69). Consequently, some facilities have begun to favor the use of tubed bovine pericardial conduits as alternatives to synthetic materials (81). Although Oizumi et al. suggested that intraoperative heparin and postoperative anticoagulants may not be necessary for SVC reconstruction (77), many centers still advocate the use of anticoagulation therapy for a certain duration after surgery. Typically, anticoagulation is continued for at least 6 months postoperatively, allowing sufficient time for endothelial formation in the graft. However, even after 6 months, there remains a risk of thrombus formation, and if possible, considering ongoing anticoagulation therapy is advisable to mitigate this risk. Ultimately, the duration and necessity of anticoagulation therapy should be determined on an individual basis, considering the patient’s overall condition, risk factors, and surgical outcomes.
Phrenic nerve reconstruction
The phrenic nerve mainly originates from the C4 cervical nerve and descends into the thoracic cavity along the anterior surface of the anterior scalene muscle. It passes between the mediastinal pleura and the pericardium and continues anteriorly into the thoracic cavity until it reaches the diaphragm. Owing to its anatomical position, the phrenic nerve is susceptible to tumor invasion in cases of locally advanced TETs. Invasive thymomas have been reported to involve the phrenic nerve in approximately 33% of cases (82). Moreover, the occurrence of diaphragmatic paralysis subsequent to concurrent resection of the phrenic nerve leads to a notable 20% to 30% reduction in lung capacity (83). Previous surgical reports have indicated that 21–68% of patients with locally advanced TETs require concomitant resection of the phrenic nerve to achieve complete tumor removal (23,25,39-41).
Unilateral phrenic nerve paralysis is a frequent complication of thoracic surgery that can cause symptoms, particularly in patients with marginal lung function (84,85). Schoeller et al. suggested that rapid microsurgical reconstruction of the phrenic nerve might be the optimal treatment for post phrenic nerve complications, especially in cases of nerve transection during surgery (86). The intercostal nerve is commonly used for phrenic nerve reconstruction, but there have been reports of using other nerves, such as the collateral mitral branch of the accessory nerve and the peroneal nerve. Additionally, techniques, such as anastomosis with 8-0 suture and fibrin glue, have been employed for nerve reconstruction (86,87). Nerve reconstruction is generally considered less invasive than diaphragm plication, which involves surgical tightening of the diaphragm muscle. However, nerve reconstruction is typically recommended in cases of short-term paralysis after nerve transection only. This is because prolonged paralysis can lead to the collapse and degeneration of the motor endplates, which are necessary for muscle innervation. The effectiveness of nerve reconstruction diminishes when the motor endplates are irreversibly denervated. Therefore, early intervention with nerve reconstruction is crucial for maximizing the chances of successful reinnervation and functional recovery of the diaphragm. According to Schoeller et al., immediate microsurgical phrenic nerve repair is recommended when phrenic nerve palsy is diagnosed due to complications from resection or surgery. However, certain conditions must be satisfied for this approach (86).
- Sufficient timeframe: nerves regenerate at a rate of approximately 1 mm per day from the proximal nerve junction site to the motor endplate of the diaphragm. Therefore, a timeframe that allows complete reinnervation is required.
- Thoracotomy for other reasons: the thoracotomy, a surgical incision into the chest wall, should be performed for reasons unrelated to nerve reconstruction.
- General condition of the patient: the patient should be in a stable general condition that allows at least 30 min of additional operative time for nerve reconstruction without an increased risk to well-being.
By fulfilling these conditions, immediate microsurgical phrenic nerve repair can be considered a treatment option for phrenic nerve palsy in cases of complications related to resection or surgery. Recovery of diaphragmatic motion following nerve reconstruction can take a variable amount of time, typically ranging from 3 to 12 months (88). The success rate of reinnervation using nerve grafts is reported to be 66.7% (88); the success of reinnervation depends on several factors (89), including the interval between nerve resection and reconstruction, the shorter the time between nerve resection and reconstruction, the higher the likelihood of successful reinnervation. Early intervention allows for better nerve regeneration and functional recovery. - Mechanical tension at the anastomosis: tension applied to the nerve anastomosis plays a role in successful reinnervation. Appropriate tensioning ensures optimal alignment and promotes nerve regeneration.
- Sharpness of the injury: the nature and severity of the nerve injury can influence the likelihood of successful reinnervation. Nerves that have been sharply cut or cleanly transected tend to have better regenerative potential than those with more extensive damage.
Aprile et al. and Yano et al. (90,91) have reported that, for TETs at or beyond the Masaoka III stage, retaining the integrity of the phrenic nerve, even when the nerve is involved, leads to enhanced functional prognosis, without discernible variations in long-term survival rates. Accordingly, the preservation of the phrenic nerve is deemed significant in the context of surgical intervention, even in cases of advanced disease. However, that the surgical management of locally advanced TETs could potentially entail resection of both phrenic nerves. Various methods have been employed to address this complication, including positive pressure ventilation, permanent ventilatory support, diaphragmatic pacing, and diaphragm plication (92). Bilateral diaphragmatic plication for bilateral phrenic nerve palsy may preserve lung capacity in the acute phase. However, the long-term effects of the procedure remain poorly understood. Diaphragmatic nerve reconstruction may be the only curative treatment for nerve palsy. It aims to restore diaphragmatic functionality and enable normal breathing. Case reports have documented successful outcomes in patients with bilateral diaphragmatic nerve palsy following diaphragmatic nerve reconstruction and diaphragm plication procedures (93).
Surgical outcomes of locally advanced TETs
There is limited literature available on the surgical treatment of locally advanced TETs following induction therapy, particularly regarding short-term postoperative outcomes. This chapter aimed to shed light on short-term postoperative outcomes following induction therapy for locally advanced TETs. Table 3 lists reports of the surgical treatment of locally advanced TETs after induction therapy since 2000, including the results of postoperative complications. The total number of cases was 466, and the reported incidence of postoperative complications varied widely from 4.8–42% (19-21,23,25,34,38-44). Only few reports have described the grading of complications. However, one must note that locally advanced TETs are a heterogeneous group, and the use of different induction therapy approaches, including radiotherapy and chemotherapy, further complicates direct comparisons between studies. Upon reviewing these reports, it is evident that complications such as pneumonia, atelectasis, atrial fibrillation, other arrhythmias, postoperative bleeding, and postoperative wound infection are relatively common. These complications are expected given the highly invasive nature of the surgery and resection of the surrounding organs, which is often necessary in the treatment of locally advanced TETs. Additionally, the relatively high frequency of respiratory failure and acute respiratory distress syndrome (ARDS) attributed to phrenic or recurrent nerve palsy and potential exacerbation of MG, which may be characteristic of this particular patient population. Mineo et al. reported a mild correlation between the incidence of postoperative complications and factors, such as the presence of complications, reconstruction of surrounding organs, and complete tumor resection (39). In contrast, Yu et al. found that the group that received preoperative chemoradiotherapy had a significantly higher rate of surgical site infection than the group that did not receive such treatment. However, no significant differences were found in the postoperative chest tube drainage time, hospitalization time, postoperative arrhythmia, or incidence of pneumonia between the groups that underwent chemoradiotherapy plus surgery and those that underwent surgery alone (44).
Table 3
| Author | Year | Study design | Total (n) | Morbidity rate | Details of complications | Mortality rate |
|---|---|---|---|---|---|---|
| Kim (19) | 2004 | Prospective | 22 | 1 (4.5%) | ARDS | 0% |
| Lucchi (20) | 2005 | Prospective | 36 | > G3: 0% | – | 0% |
| Wright (38) | 2008 | Retrospective | 10 | 2 (20%) | Cardiac tamponade, ARDS | 0% |
| Kunitoh (21) | 2010 | Prospective | 21 | 1 (4.8%) | Pulmonary infarction | 0% |
| Mineo (39) | 2010 | Retrospective | 33 | 14 (42%) | Acute pneumonia (n=6) and respiratory failure requiring tracheal intubation (n=4) | N/A |
| Cardillo (40) | 2010 | Retrospective | 31 | 9 (29%) | Pulmonary embolism (n=1), postoperative bleeding (n=1), pulmonary infections (n=2) and wound infections (n=5). One patient experienced adult respiratory distress syndrome after surgical resection and required prolonged hospitalisation | 0% |
| Rea (41) | 2011 | Retrospective | 38 | 4 (5.3%) | A wound infection with sternal dehiscence, a bronchopleural fistula after pneumonectomy, pneumonia, and respiratory insufficiency in a patient with myasthenia gravis and phrenic nerve resection | 0% |
| Korst (23) | 2014 | Prospective | 22 | 8 (36.4%) | Pneumonia, pulmonary infiltrate, hemothorax, mucous plugging, aspiration, pleural effusion, intraoperative cardiac arrest, multiple organ failure, neutropenia, dressler syndrome (n=2), atrial fibrillation (n=3) | 2 (9%) |
| Shintani (25) | 2015 | Retrospective | 16 | 6 (37.5%) | Bleeding, cardiac depolarization, cardiac tamponade, chylothorax, and bilateral recurrent nerve palsy | 0% |
| Cardillo (42) | 2016 | Retrospective | 108 | 21 (19.4%) | Mostly anemia requiring blood transfusion or atelectasis | 2 (1.8%) |
| Kanzaki (43) | 2019 | Retrospective | 29 | 6 (21%) | Myasthenic crisis, pneumonia, postoperative bleeding, recurrent nerve palsy, cardiac hernia, chylothorax, wound infection, respiratory failure | 0% |
| Yu (44) | 2021 | Retrospective | 19 | – | Arrhythmia, pneumonia, surgical site infection, hoarseness, myasthenic crisis, postoperative bleeding, empyema, pulmonary thromboembolism | 0% |
| Wang (34) | 2021 | Retrospective | 81 | 12 (14.8%) | Including four cases of bleeding, five cases of hydrothorax, two cases of atelectasis and one case of unhealing wounds | 0% |
ARDS, acute respiratory distress syndrome; G3, grade 3; N/A, not available.
Surprisingly, a low perioperative mortality rate has been observed in patients treated with induction therapy followed by surgery for locally advanced TETs. Most studies have reported a perioperative mortality rate of 0%. However, Cardillo et al. conducted a retrospective study of 108 patients and reported perioperative mortality in two cases (1.8%) (42). Similarly, Korst et al. conducted a prospective study and reported two cases (9%) of perioperative mortality (23). Among the cases reported by Korst et al., one was a patient who experienced respiratory failure due to aspiration pneumonia after combined resection of the phrenic and vagus nerves, and the other was a case of cardiac arrest and multi-organ failure during debulking surgery in a patient with clear infiltration of the ascending aorta (23). As locally advanced TETs often require resection of the surrounding organs, the decision to proceed with surgery should be carefully evaluated, taking into consideration the patient’s general condition and other relevant factors.
The postoperative survival outcomes are presented in Table 2. The 5-year OS, 5-year recurrence-free survival (RFS), and 10-year OS rates after induction therapy for locally advanced TETs ranged from 44.9% to 100%, 29.5% to 83%, and 19.9% to 90%, respectively (18-25,33,34,37-44). Although there is considerable variation among the reports, the overall results are generally favorable. These outcomes reflect the oncological characteristics of thymomas. Distinguishing between thymomas and thymic carcinomas in TETs can be challenging, particularly when making histological diagnoses prior to treatment. Multiple factors need to be considered, ranging from the initial diagnosis to the implementation of induction therapy and the determination of the appropriate surgical approach.
Limitation
This narrative review has several limitations. Firstly, it should be noted that this is not a comprehensive systematic review encompassing all available research on locally advanced TETs. Furthermore, we did not conduct a specific comparison of the overall quality of the research included in our review. Our focus was on incorporating relevant literature pertaining to induction therapy, combined resection of surrounding organs, surgical approach, revascularization, diaphragmatic nerve reconstruction, and short-term postoperative outcomes, with the aim of providing guidance for perioperative management of locally advanced TETs.
Conclusions
In locally advanced TETs, the short-term postoperative outcomes of surgical treatment after induction therapy are generally acceptable, with a relatively low mortality rate. However, surgical resection of the surrounding organs is often necessary, and effective management of postoperative complications is crucial. In certain cases, incomplete resection occurs, particularly when the tumor invades the pulmonary trunk or aorta. Therefore, the complexities associated with resecting these large vessels must be carefully considered, keeping in mind the patient’s overall condition and treatment outcomes. We anticipate future advancements in minimally invasive surgical approaches, surgical techniques, and perioperative pharmacotherapies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Masatsugu Hamaji) for the series “Locally Advanced Thymic Epithelial Tumors” published in Mediastinum. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://med.amegroups.com/article/view/10.21037/med-23-24/rc
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-23-24/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-23-24/coif). The series “Locally Advanced Thymic Epithelial Tumors” was commissioned by the editorial office without any funding or sponsorship. F.T. reports receiving research grants from Boehringer Ingelheim Japan, Ono Pharmaceutical, Taiho Pharmaceutical, Eli Lilly Japan, and Chugai Pharmaceutical. FT also reports receiving consulting fees from AstraZeneca, Chugai Pharmaceutical, and Ono Pharmaceutical, as well as receiving payment for lectures from MSD, Bristol-Myers Squibb, Boehringer Ingelheim Japan, Ono Pharmaceutical, Johnson & Johnson, Covidien Japan, Taiho Pharmaceutical, Eli Lilly Japan, AstraZeneca, Chugai Pharmaceutical, Kyowa-Kirin, Takeda Pharmaceutical, Pfizer, Olympus, Stryker, and Intuitive Japan. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol 2010;5:S260-5. [Crossref] [PubMed]
- Huang J, Ahmad U, Antonicelli A, et al. Development of the international thymic malignancy interest group international database: an unprecedented resource for the study of a rare group of tumors. J Thorac Oncol 2014;9:1573-8. [Crossref] [PubMed]
- Girard N, Ruffini E, Marx A, et al. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v40-55. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. Thymomas and Thymic Carcinomas. Version 2. 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/thymic.pdf
- Brierley JD, Gospodarowicz MK, Wittekind C. editors. Union for International Cancer Control (UICC) TNM Classification of Malignant Tumors, 8th ed. Hoboken, New Jersey: Wiley-Blackwell, 2017.
- Congedo E, Aceto P, Cardone A, et al. Perioperative management of thymectomy. Ann Ital Chir 2007;78:367-70. [PubMed]
- Levin H, Bursztein S, Heifetz M. Cardiac arrest in a child with an anterior mediastinal mass. Anesth Analg 1985;64:1129-30. [Crossref] [PubMed]
- Northrip DR, Bohman BK, Tsueda K. Total airway occlusion and superior vena cava syndrome in a child with an anterior mediastinal tumor. Anesth Analg 1986;65:1079-82. [Crossref] [PubMed]
- Béchard P, Létourneau L, Lacasse Y, et al. Perioperative cardiorespiratory complications in adults with mediastinal mass: incidence and risk factors. Anesthesiology 2004;100:826-34; discussion 5A. [Crossref] [PubMed]
- Daum P, Smelt J, Ibrahim IR. Perioperative management of myasthenia gravis. BJA Educ 2021;21:414-9. [Crossref] [PubMed]
- Ahmad U, Huang J. Current readings: The most influential and recent studies involving surgical management of thymoma. Semin Thorac Cardiovasc Surg 2013;25:144-9. [Crossref] [PubMed]
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of1320 patients from Japan. Ann Thorac Surg 2003;76:878-84. [Crossref] [PubMed]
- Detterbeck FC, Parsons AM. Thymic tumors. Ann Thorac Surg 2004;77:1860-9. [Crossref] [PubMed]
- Blumberg D, Port JL, Weksler B, et al. Thymoma: a multivariate analysis of factors predicting survival. Ann Thorac Surg 1995;60:908-13; discussion 914. [Crossref] [PubMed]
- Chiappetta M, Aprile V, Lococo F, et al. Prognostic factors for survival in advanced thymomas: The role of the number of involved structures. J Surg Oncol 2021;124:858-66. [Crossref] [PubMed]
- Giaccone G, Musella R, Bertetto O, et al. Cisplatin-containing chemotherapy in the treatment of invasive thymoma: report of five cases. Cancer Treat Rep 1985;69:695-7. [PubMed]
- Ahmad U, Huang J. Induction Therapy for Thymoma. Thorac Surg Clin 2016;26:325-32. [Crossref] [PubMed]
- Venuta F, Rendina EA, Longo F, et al. Long-term outcome after multimodality treatment for stage III thymic tumors. Ann Thorac Surg 2003;76:1866-72; discussion 1872. [Crossref] [PubMed]
- Kim ES, Putnam JB, Komaki R, et al. Phase II study of a multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation therapy, and consolidation chemotherapy for unresectable malignant thymomas: final report. Lung Cancer 2004;44:369-79. [Crossref] [PubMed]
- Lucchi M, Ambrogi MC, Duranti L, et al. Advanced stage thymomas and thymic carcinomas: results of multimodality treatments. Ann Thorac Surg 2005;79:1840-4. [Crossref] [PubMed]
- Kunitoh H, Tamura T, Shibata T, et al. A phase II trial of dose-dense chemotherapy, followed by surgical resection and/or thoracic radiotherapy, in locally advanced thymoma: report of a Japan Clinical Oncology Group trial (JCOG 9606). Br J Cancer 2010;103:6-11. [Crossref] [PubMed]
- Park S, Ahn MJ, Ahn JS, et al. A prospective phase II trial of induction chemotherapy with docetaxel/cisplatin for Masaoka stage III/IV thymic epithelial tumors. J Thorac Oncol 2013;8:959-66. [Crossref] [PubMed]
- Korst RJ, Bezjak A, Blackmon S, et al. Neoadjuvant chemoradiotherapy for locally advanced thymic tumors: a phase II, multi-institutional clinical trial. J Thorac Cardiovasc Surg 2014;147:36-44, 46.e1.
- Fan XW, Yang Y, Wang HB, et al. Intensity Modulated Radiation Therapy Plus Etoposide/Cisplatin for Patients With Limited Advanced Unresectable Thymic Epithelial Tumors: A Prospective Phase 2 Study. Int J Radiat Oncol Biol Phys 2020;107:98-105. [Crossref] [PubMed]
- Shintani Y, Inoue M, Kawamura T, et al. Multimodality treatment for advanced thymic carcinoma: outcomes of induction therapy followed by surgical resection in 16 cases at a single institution. Gen Thorac Cardiovasc Surg 2015;63:159-63. [Crossref] [PubMed]
- Shintani Y, Funaki S, Ose N, et al. Surgical management of thymic epithelial tumors. Surg Today 2021;51:331-9. [Crossref] [PubMed]
- Ruffini E, Detterbeck F, Van Raemdonck D, et al. Thymic carcinoma: a cohort study of patients from the European society of thoracic surgeons database. J Thorac Oncol 2014;9:541-8. [Crossref] [PubMed]
- Weksler B, Dhupar R, Parikh V, et al. Thymic carcinoma: a multivariate analysis of factors predictive of survival in 290 patients. Ann Thorac Surg 2013;95:299-303. [Crossref] [PubMed]
- Hishida T, Nomura S, Yano M, et al. Long-term outcome and prognostic factors of surgically treated thymic carcinoma: results of 306 cases from a Japanese Nationwide Database Study. Eur J Cardiothorac Surg 2016;49:835-41. [Crossref] [PubMed]
- Kondo K, Monden Y. Lymphogenous and hematogenous metastasis of thymic epithelial tumors. Ann Thorac Surg 2003;76:1859-64; discussion 1864-5. [Crossref] [PubMed]
- Hamaji M, Kojima F, Omasa M, et al. A meta-analysis of debulking surgery versus surgical biopsy for unresectable thymoma. Eur J Cardiothorac Surg 2015;47:602-7. [Crossref] [PubMed]
- Leuzzi G, Rocco G, Ruffini E, et al. Multimodality therapy for locally advanced thymomas: A propensity score-matched cohort study from the European Society of Thoracic Surgeons Database. J Thorac Cardiovasc Surg 2016;151:47-57.e1. [Crossref] [PubMed]
- Wei Y, Gu Z, Shen Y, et al. Preoperative induction therapy for locally advanced thymic tumors: a retrospective analysis using the ChART database. J Thorac Dis 2016;8:665-72. [Crossref] [PubMed]
- Wang S, Jiang J, Gao J, et al. Induction Therapy Followed by Surgery for Unresectable Thymic Epithelial Tumours. Front Oncol 2021;11:791647. [Crossref] [PubMed]
- Kondo K. Optimal therapy for thymoma. J Med Invest 2008;55:17-28. [Crossref] [PubMed]
- Hamaji M, Allen MS, Cassivi SD, et al. The role of surgical management in recurrent thymic tumors. Ann Thorac Surg 2012;94:247-54; discussion 254. [Crossref] [PubMed]
- Yokoi K, Matsuguma H, Nakahara R, et al. Multidisciplinary treatment for advanced invasive thymoma with cisplatin, doxorubicin, and methylprednisolone. J Thorac Oncol 2007;2:73-8. [Crossref] [PubMed]
- Wright CD, Choi NC, Wain JC, et al. Induction chemoradiotherapy followed by resection for locally advanced Masaoka stage III and IVA thymic tumors. Ann Thorac Surg 2008;85:385-9. [Crossref] [PubMed]
- Mineo TC, Mineo D, Onorati I, et al. New predictors of response to neoadjuvant chemotherapy and survival for invasive thymoma: a retrospective analysis. Ann Surg Oncol 2010;17:3022-9. [Crossref] [PubMed]
- Cardillo G, Carleo F, Giunti R, et al. Predictors of survival in patients with locally advanced thymoma and thymic carcinoma (Masaoka stages III and IVa). Eur J Cardiothorac Surg 2010;37:819-23. [Crossref] [PubMed]
- Rea F, Marulli G, Di Chiara F, et al. Multidisciplinary approach for advanced stage thymic tumors: long-term outcome. Lung Cancer 2011;72:68-72. [Crossref] [PubMed]
- Cardillo G, Lucchi M, Marulli G, et al. Induction therapy followed by surgical resection in Stage-III thimic epithelial tumors: Long-term results from a multicentre analysis of 108 cases. Lung Cancer 2016;93:88-94. [Crossref] [PubMed]
- Kanzaki R, Kanou T, Ose N, et al. Long-term outcomes of advanced thymoma in patients undergoing preoperative chemotherapy or chemoradiotherapy followed by surgery: a 20-year experience. Interact Cardiovasc Thorac Surg 2019;28:360-7. [Crossref] [PubMed]
- Yu Z, Yu L, Yu T, et al. Surgical feasibility and long-term outcome of superior vena cava replacement for advanced thymoma in patients undergoing preoperative chemotherapy or chemoradiotherapy. Thorac Cancer 2021;12:1074-83. [Crossref] [PubMed]
- Yamato H, Funaki S, Shimamura K, et al. Salvage surgery for stage IVa thymic carcinoma combined with aortic arch resection - case report. J Cardiothorac Surg 2020;15:305. [Crossref] [PubMed]
- Kurata A, Saji H, Ikeda N, et al. Intracaval and intracardiac extension of invasive thymoma complicated by superior and inferior vena cava syndrome. Pathol Int 2013;63:56-62. [Crossref] [PubMed]
- De Giacomo T, Patella M, Mazzesi G, et al. Successful resection of thymoma directly invading the right atrium under cardiopulmonary bypass. Eur J Cardiothorac Surg 2015;48:332-3. [Crossref] [PubMed]
- Momozane T, Inoue M, Shintani Y, et al. Trimodality Therapy for an Advanced Thymic Carcinoma With Both Aorta and Vena Cava Invasion. Ann Thorac Surg 2016;102:e139-41. [Crossref] [PubMed]
- Ried M, Neu R, Schalke B, et al. Radical surgical resection of advanced thymoma and thymic carcinoma infiltrating the heart or great vessels with cardiopulmonary bypass support. J Cardiothorac Surg 2015;10:137. [Crossref] [PubMed]
- Chen L, Zhu X, Zhu R, et al. Cardiopulmonary bypass does not consequentially contribute to postoperative distant metastasis of giant refractory thoracic tumors: A retrospective study with long-term follow-up. Thorac Cancer 2021;12:2990-5. [Crossref] [PubMed]
- Mastromarino MG, Bacchin D, Aprile V, et al. Unradical Surgery for Locally-Advanced Thymoma: Is it time to evolve Perspectives? Lung Cancer 2023;180:107214. [Crossref] [PubMed]
- Grunenwald D, Spaggiari L. Transmanubrial osteomuscular sparing approach for apical chest tumors. Ann Thorac Surg 1997;63:563-6. [Crossref] [PubMed]
- Korst RJ, Burt ME. Cervicothoracic tumors: results of resection by the "hemi-clamshell" approach. J Thorac Cardiovasc Surg 1998;115:286-94; discussion 294-5. [Crossref] [PubMed]
- Shiono H, Sakamoto T, Sakurai T. Minimally invasive anterior chest wall lifting technique for thoracoscopic mediastinal approach. Gen Thorac Cardiovasc Surg 2016;64:564-7. [Crossref] [PubMed]
- Suda T, Kaneda S, Hachimaru A, et al. Thymectomy via a subxiphoid approach: single-port and robot-assisted. J Thorac Dis 2016;8:S265-71. [PubMed]
- Tomulescu V, Popescu I. Unilateral extended thoracoscopic thymectomy for nontumoral myasthenia gravis--a new standard. Semin Thorac Cardiovasc Surg 2012;24:115-22. [Crossref] [PubMed]
- Suda T, Sugimura H, Tochii D, et al. Single-port thymectomy through an infrasternal approach. Ann Thorac Surg 2012;93:334-6. [Crossref] [PubMed]
- Suda T, Hachimaru A, Tochii D, et al. Video-assisted thoracoscopic thymectomy versus subxiphoid single-port thymectomy: initial results†. Eur J Cardiothorac Surg 2016;49:i54-8. [PubMed]
- Suda T, Ashikari S, Tochii D, et al. Dual-port thymectomy using subxiphoid approach. Gen Thorac Cardiovasc Surg 2014;62:570-2. [Crossref] [PubMed]
- Rueckert J, Swierzy M, Badakhshi H, et al. Robotic-assisted thymectomy: surgical procedure and results. Thorac Cardiovasc Surg 2015;63:194-200. [Crossref] [PubMed]
- Petersen RH. Video-assisted thoracoscopic thymectomy using 5-mm ports and carbon dioxide insufflation. Ann Cardiothorac Surg 2016;5:51-5. [PubMed]
- Xu H, Liu D, Li Y, et al. The Outcomes of Subxiphoid Thoracoscopic Versus Video-Assisted Thoracic Surgery for Thymic Diseases. J Laparoendosc Adv Surg Tech A 2020;30:508-13. [Crossref] [PubMed]
- Zielinski M, Czajkowski W, Gwozdz P, et al. Resection of thymomas with use of the new minimally-invasive technique of extended thymectomy performed through the subxiphoid-right video-thoracoscopic approach with double elevation of the sternum. Eur J Cardiothorac Surg 2013;44:e113-9; discussion e119. [Crossref] [PubMed]
- Wang X, Aramini B, Xu H, et al. Thymectomy with angioplasty through a thoracoscopic subxiphoid approach with double elevation of the sternum in Masaoka stage III thymoma. JTCVS Tech 2021;8:208-10. [Crossref] [PubMed]
- Kang CH, Na KJ, Song JW, et al. The robotic thymectomy via the subxiphoid approach: technique and early outcomes. Eur J Cardiothorac Surg 2020;58:i39-43. [Crossref] [PubMed]
- Toker A, Awori Hayanga JW, Dhamija A, et al. Superior vena cava reconstruction in mediastinal tumors. Am J Surg 2021;222:294-6. [Crossref] [PubMed]
- Leo F, Bellini R, Conti B, et al. Superior vena cava resection in thoracic malignancies: does prosthetic replacement pose a higher risk? Eur J Cardiothorac Surg 2010;37:764-9. [Crossref] [PubMed]
- Spaggiari L, Galetta D. Superior vena cava resection. In: Dienemann HC, Hoffmann H, Detterbeck FC, editors. Chest Surgery. Berlin: Springer Berlin, Heidelberg, 2014:341-50.
- Spaggiari L, Leo F, Veronesi G, et al. Superior vena cava resection for lung and mediastinal malignancies: a single-center experience with 70 cases. Ann Thorac Surg 2007;83:223-9; discussion 229-30. [Crossref] [PubMed]
- Lanuti M, De Delva PE, Gaissert HA, et al. Review of superior vena cava resection in the management of benign disease and pulmonary or mediastinal malignancies. Ann Thorac Surg 2009;88:392-7. [Crossref] [PubMed]
- Wright CD. Extended resections for thymic malignancies. J Thorac Oncol 2010;5:S344-7. [Crossref] [PubMed]
- Maurizi G, Poggi C, D'Andrilli A, et al. Superior Vena Cava Replacement for Thymic Malignancies. Ann Thorac Surg 2019;107:386-92. [Crossref] [PubMed]
- Leo F, Della Grazia L, Tullii M, et al. Hemodynamic instability during superior vena cava crossclamping: predictors, management, and clinical consequences. J Thorac Cardiovasc Surg 2007;133:1105-6. [Crossref] [PubMed]
- Jones DR. Technique of superior vena cava resection for lung carcinomas. Operative Techniques in Thoracic and Cardiovascular Surgery 2008;13:274-82. [Crossref]
- D'Andrilli A, De Cecco CN, Maurizi G, et al. Reconstruction of the superior vena cava by biologic conduit: assessment of long-term patency by magnetic resonance imaging. Ann Thorac Surg 2013;96:1039-45. [Crossref] [PubMed]
- Nawashiro A, Takenaka M, Matsumiya H, et al. A technique of revascularization in mediastinal tumor surgery with superior vena cava resection. Jpn J Chest Surg 2021;35:207-12. [Crossref]
- Oizumi H, Suzuki K, Banno T, et al. Patency of grafts after total resection and reconstruction of the superior vena cava for thoracic malignancy. Surg Today 2016;46:1421-6. [Crossref] [PubMed]
- Shintani Y, Ohta M, Minami M, et al. Long-term graft patency after replacement of the brachiocephalic veins combined with resection of mediastinal tumors. J Thorac Cardiovasc Surg 2005;129:809-12. [Crossref] [PubMed]
- Sekine Y, Suzuki H, Saitoh Y, et al. Prosthetic reconstruction of the superior vena cava for malignant disease: surgical techniques and outcomes. Ann Thorac Surg 2010;90:223-8. [Crossref] [PubMed]
- Nakano T, Endo S, Kanai Y, et al. Surgical outcomes after superior vena cava reconstruction with expanded polytetrafluoroethylene grafts. Ann Thorac Cardiovasc Surg 2014;20:310-5. [Crossref] [PubMed]
- Bertolaccini L, Prisciandaro E, Galetta D, et al. Outcomes and Safety Analysis in Superior Vena Cava Resection for Extended Thymic Epithelial Tumors. Ann Thorac Surg 2021;112:271-7. [Crossref] [PubMed]
- Marulli G, Margaritora S, Lucchi M, et al. Surgical treatment of recurrent thymoma: is it worthwhile?†. Eur J Cardiothorac Surg 2016;49:327-32. [Crossref] [PubMed]
- Shields TW. Diaphragmatic function, diaphragmatic paralysis, and elevation of the diaphragm. In: Shields TW, Locicero J, Ponn RB, editors. General Thoracic Surgery. 6th ed. Vol I. Philadelphia, PA: Lippincott, Williams and Wilkins; 2004:740-5.
- Tripp HF, Bolton JW. Phrenic nerve injury following cardiac surgery: a review. J Card Surg 1998;13:218-23. [Crossref] [PubMed]
- de Leeuw M, Williams JM, Freedom RM, et al. Impact of diaphragmatic paralysis after cardiothoracic surgery in children. J Thorac Cardiovasc Surg 1999;118:510-7. [Crossref] [PubMed]
- Schoeller T, Ohlbauer M, Wechselberger G, et al. Successful immediate phrenic nerve reconstruction during mediastinal tumor resection. J Thorac Cardiovasc Surg 2001;122:1235-7. [Crossref] [PubMed]
- Yang ML, Li JJ, Zhang SC, et al. Functional restoration of the paralyzed diaphragm in high cervical quadriplegia via phrenic nerve neurotization utilizing the functional spinal accessory nerve. J Neurosurg Spine 2011;15:190-4. [Crossref] [PubMed]
- Kaufman MR, Elkwood AI, Rose MI, et al. Reinnervation of the paralyzed diaphragm: application of nerve surgery techniques following unilateral phrenic nerve injury. Chest 2011;140:191-7. [Crossref] [PubMed]
- Mackinnon SE, Dellon AL. Surgery of the Peripheral Nerve. New York: Thieme Medical Publishers; 1988:89-93.
- Aprile V, Bertoglio P, Korasidis S, et al. Nerve-Sparing Surgery in Advanced Stage Thymomas. Ann Thorac Surg 2019;107:878-84. [Crossref] [PubMed]
- Yano M, Sasaki H, Moriyama S, et al. Preservation of phrenic nerve involved by stage III thymoma. Ann Thorac Surg 2010;89:1612-9. [Crossref] [PubMed]
- Stolk J, Versteegh MI. Long-term effect of bilateral plication of the diaphragm. Chest 2000;117:786-9. [Crossref] [PubMed]
- Shinohara S, Yamada T, Ueda M, et al. Phrenic Nerve Reconstruction and Bilateral Diaphragm Plication After Lobectomy. Ann Thorac Surg 2017;104:e9-e11. [Crossref] [PubMed]
Cite this article as: Takenaka M, Kuroda K, Tanaka F. Perioperative management and postoperative outcomes of locally advanced thymic epithelial tumors: a narrative review. Mediastinum 2024;8:7.


