
Pathological snapshots of thymic epithelial tumors with invasion into neighboring structures: preparing for the forthcoming revision of the TNM classification
Introduction
Pathological staging is fundamental for the clinical treatment of patients, including those with thymic epithelial tumors (TETs). The International Thymic Malignancy Interest Group (ITMIG), the first international association for thymic malignancies and the current and undoubtedly future leader in dealing with these rare cancers, first selected the Masaoka-Koga staging system for staging TETs (1,2), considering that it had been the most widely accepted system (3). Subsequently, the ITMIG and the International Association of the Study of Lung Cancer (IASLC) proposed the first international and evidence-based stage classification based on statistical analyses of a large retrospective database (4). It was approved by the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC) and was included in the 8th edition of the tumor, node, metastasis (TNM) classification (TNM-8) of malignant tumors (5). Accordingly, the current (5th) World Health Organization (WHO) classification for TETs states that use of the TNM system is mandatory and that use of the Masaoka-Koga system is optional (6). However, most institutions use both systems in practice, and clinicians seem to prefer the Masaoka-Koga system when considering postoperative radiation therapy (7).
Both systems define stages based on the same concept; that is, to what extent the tumor invades the surrounding structures, although the defining factors are slightly different. When staging TETs, pathologists evaluate the presence or absence of every defining factor to determine the final stage. The term “locally advanced”, the topic of this series, generally indicates stages III and IVa in the Masaoka-Koga system.
The ITMIG has published many educational articles about staging TETs, which are highly appreciated (3,4,7-10); however, articles with actual histological images of TETs exhibiting invasion into the peri-thymic structures are relatively rare. Thus, in this review, we attempt to provide pathological snapshots of findings that affect either the Masaoka-Koga system or TNM-8. To this end, we describe all the findings that affect the pathological stages of the two systems, not limited to those corresponding to “locally advanced TETs” for a complete reference. Regarding this, the article by Detterbeck et al. was particularly helpful in clarifying the definitions of potentially ambiguous terms used in the Masaoka-Koga system (3). The new 9th edition of the TNM classification for TETs will be available in January 2024. Thus, reconfirming the current status of pathological evaluation of TETs might help readers prepare for the upcoming revision of the TNM system and to clarify similarities and differences between the 8th and 9th editions.
Tumor subtype, especially whether the tumor is a thymoma or thymic carcinoma, also influences treatment decisions (https://www.nccn.org/home). Recent studies, particularly those conducted as part of The Cancer Genome Atlas (TCGA) project (11), have advanced our biological understanding of TETs. This review also introduces these recent discoveries, delineating each TET subtype and describing the features that allow discrimination between thymomas and thymic carcinomas.
Microscopic trans-capsular invasion
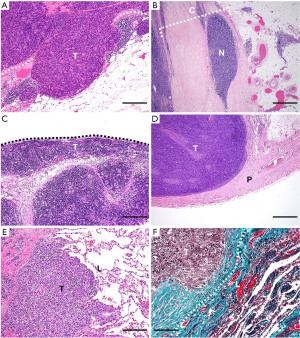
In the Masaoka-Koga system, microscopic trans-capsular invasion (Figure 1A) is designated as stage II (1,2). However, this finding does not affect stage in the TNM-8 (5) because it was found to have no impact on patient outcomes in a large dataset (4,9). This decision is histologically supported because the thymus is not physiologically surrounded by a fibrous capsule. Despite this, many surgeons believe that the presence or absence of capsular invasion should be a factor dividing the pT category (7). Because the fibrous capsule is intraoperatively recognizable, surgeons may assume that the pathological absence of capsular invasion indicates that the tumor extent is macroscopically and microscopically the same, and they may be convinced that the tumor is completely resected without chance of recurrence. When determining trans-capsular invasion of TETs, pathologists should consider the consensus definition by ITMIG (3), which determines trans-capsular invasion when the tumor has a fibrous capsule in the corresponding areas, penetrates the capsule, and reaches the surrounding peri-thymic fat. In other words, a simple lack of a tumor capsule, separate nodules within the capsule (Figure 1B), and direct intracapsular spread, do not indicate trans-capsular invasion. The Masaoka-Koga system separates stage II into IIa and IIb. The original description was whether the trans-capsular invasion was microscopic (IIa) or macroscopic (IIb) (1), but subdivision by the distance from the capsule [≤3 mm (IIa) or > 3 mm (IIb)] is accepted by ITMIG (3).
Invasion of the mediastinal pleura
Direct invasion of the mediastinal pleura (Figure 1C) defines a tumor as stage III in the Masaoka-Koga system and as pT1b in the TNM-8. No data, except that from the Japanese Association for Research in the Thymus (JART), indicate the prognostic impact of this factor, and it has been tentatively separated as “b” within the pT1 category (4). One concern is that it is not easy to histologically identify mediastinal pleura, especially when the specimen orientation is poor or an inflammatory reaction involving the pleura occurs. This problem has been repeatedly noted (4,9), and it will be interesting to see how the forthcoming TNM classification deals with this issue.
Direct involvement of the pericardium
Direct pericardial invasion defines stage III in the Masaoka-Koga system and pT2 in the TNM-8 (Figure 1D). In addition to the observed difference in disease recurrence among stages I, II, and III (5%, 18%, and 32%, respectively) (4,9), because pericardial tumor invasion requires partial resection and subsequent repair of the pericardium, it would be clinically reasonable to adopt an independent T category for this finding. Histological recognition of pericardial tumor invasion is generally straightforward because this structure is distinct. However, if the resected area is small, the pericardium can be missed unless specimen orientation is adequately addressed.
Direct invasion into the lung
Pulmonary invasion corresponds to stage III in the Masaoka-Koga system and pT3 in the TNM-8 (Figure 1E). The ITMIG defines pulmonary invasion as direct penetration into the outer elastin layer of the visceral pleura or the lung parenchyma (3). Thus, elastin staining highlighting elastic fibers is helpful in controversial cases (Figure 1F).
Direct invasion into the brachiocephalic vein or superior vena cava
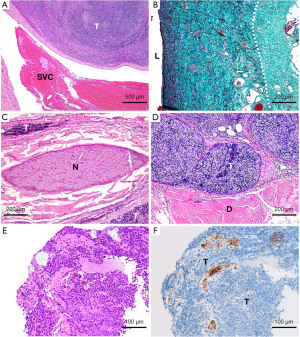
Direct invasion into the neighboring large veins defines stage III in the Masaoka-Koga system and pT3 in the TNM-8 (Figure 2A). The ITMIG defines direct invasion as an invasion into or penetration through major vascular structures (3). Large veins consist of intima, media, and adventitia. Because it is challenging to differentiate the outermost part of the adventitia from the surrounding connective tissues, we assume that pathologists practically determine large vein invasion when the tumor invades smooth muscle cells or elastic layers of the adventitia (i.e., not the outermost but a more inner part) (Figure 2B).
Invasion into the phrenic or vagus nerve
Invasion into the phrenic or vagus nerve defines stage III in the Masaoka-Koga system and pT3 in the TNM-8. The original Masaoka-Koga system did not mention this feature, but the ITMIG added it later as corresponding to stage III (3). The ITMIG states that adherence alone is insufficient, and it would be reasonable that this applies both macroscopically and microscopically. However, in our personal experiences, the microscopic definition of phrenic nerve invasion seems inconsistent among pathologists; a tumor that surrounds or is almost attached to a nerve but does not definitively invade it might be interpreted by some pathologists as “nerve invasion” (Figure 2C). The ITMIG may discuss this issue when the new TNM classification is launched. The other factor that defines pT3 in the TNM-8 is involvement of the chest wall. This feature is not mentioned in the Masaoka-Koga classification but would apply to stage III. In addition, we have had a case that showed direct invasion of a thymoma into the diaphragm (Figure 2D), which is not described in either staging system. Considering that the diaphragm seems equivalent to the chest wall in this context, it would be appropriate to describe this as stage III in the Masaoka-Koga system and pT3 in the TNM-8.
Invasion into large arteries, myocardium, trachea, or esophagus
Direct involvement of the aorta, arch vessels, main pulmonary artery, myocardium, trachea, or esophagus (Figure 2E,2F) is considered pT4 in the TNM-8 because it indicates more extensive local invasion than factors corresponding to pT3. Because these structures are easily recognizable, they will be readily determined pathologically. The Masaoka-Koga system includes all of these factors within stage III.
Separate pleural or pericardial metastases
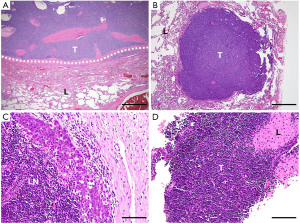
Implants on the pleura or pericardium separated from the primary tumor define stage IVa in the Masaoka-Koga system and pM1a in the TNM-8 (Figure 3A). One caution is that pulmonary intraparenchymal nodules are regarded as IVb in the Masaoka-Koga stage and M1b in the TNM-8 (Figure 3B). According to the ITMIG, pulmonary nodules that are in the lung, with a rim of normal lung between the nodule and the pleural surface, are regarded as distant metastases (3). Thus, pulmonary implants that eventually but partly invade the pulmonary parenchyma should be kept as stage IVa and pM1a, although the degree of pulmonary invasion of the implants may influence patient outcomes (12).
Lymphogenous metastases
Lymph node metastases indicate stage IVb in the Masaoka-Koga system (Figure 3C). In the TNM-8, they belong to the N category and are divided into N1 [anterior (peri-thymic) nodes] and N2 (deep intrathoracic or cervical nodes). The prognostic difference between pN1 and pN2 was not significant in analysis of a retrospective database (4,8), but we agree with the separation because, in addition to the different distance from the thymus between N1 and N2, thymic tumors can directly invade the surrounding lymph nodes (counted as pN1) (8), a situation that is biologically different from metastasis to distant lymph nodes.
Distant organ metastasis
Distant organ metastasis is classified as stage IVb in the Masaoka-Koga system and M1b in the TNM-8. The fact that thymomas rarely exhibit lymph node metastases but can metastasize to different organs (4,8), even in generally low-grade subtypes (Figure 3D), whereas thymic carcinomas can metastasize to both lymph nodes and distant organs probably reflects their biological difference.
The pathological features affecting the stage of thymic epithelial tumors is summarized as Table 1. Overall, the two most widely used systems do not consider the number of stage-determining factors that the tumor invades for staging. As to the TNM-8, this policy is based on the results of extensive statistical analyses, which did not provide compelling evidence that the number is a definite prognostic factor, as well as for simplicity (4,8). However, it is natural to think that, for example, a tumor with both pericardial and pulmonary invasion might exhibit more aggressive behavior than the tumor with (very focal) pericardial invasion alone. Thus, rather than just staging after summarizing the pathological findings, providing a synoptic report or checklist noting the presence or absence of all defining factors might be helpful for clinicians and pathologists and for future research with a more extensive patient cases. In addition, the number of metastatic (including implant) foci might become relevant, considering that treatment strategies for tumors with oligometastasis have recently been intensely discussed in thoracic cancers (13).
Table 1
| Structures | Masaoka-Koga stage classification | TNM-8 |
|---|---|---|
| Invasion | ||
| Peri-thymic fat (trans-capsular invasion) | IIa: ≤3 mm; IIb: >3 mm | pT1a |
| Mediastinal pleura | III | pT1b |
| Pericardium | III | pT2 |
| Lung | III | pT3 |
| Large veins† | III | pT3 |
| Phrenic/vagus nerve | III | pT3 |
| Chest wall | III | pT3 |
| Large arteries‡ | III | pT4 |
| Myocardium | III | pT4 |
| Trachea | III | pT4 |
| Esophagus | III | pT4 |
| Dissemination/metastasis | ||
| Pleura | IVa | pM1a |
| Pericardium | IVa | pM1a |
| Lymph nodes | IVb | pN1: peri-thymic; pN2: more distant |
| Distant organs | IVb | pM1b |
†, brachiocephalic vein, superior vena cava, and extrapericardial pulmonary veins, etc. (extrapericardial pulmonary artery is also categorized as pT3); ‡, thoracic aorta, arch vessels, intrapericardial pulmonary artery. TNM-8, the 8th edition of the TNM classification; TNM, tumor, node, metastasis.
Distinctive molecular features between thymomas and thymic carcinomas
Discrimination between thymomas and thymic carcinomas is necessary for standard treatments (https://www.nccn.org/home). Comprehensive biological investigations based on technological advancements such as next-generation sequencing and single-cell analyses have indicated that thymomas and thymic carcinomas are distinct, although some controversial cases have been reported (14). A critical discovery of TET genetics was made by Petrini et al., who first reported the GTF2I L424H mutation in most type A and AB thymomas (15). After the TNM-8 was established, a very influential study was conducted by Radovich et al. as part of a TCGA project (11). They proposed molecular subtyping of TETs, in which TETs could be divided into A (type A)-like, AB-like, B-like, and C (carcinoma)-like clusters, highlighting the distinctiveness of the carcinoma cluster (11).
The TCGA dataset has attracted many thymic researchers because of its comprehensiveness, reliability, and availability (cBioPortal: https://www.cbioportal.org/), and these researchers have also investigated under-recognized molecular features of TETs using the TCGA dataset, as well as their cohorts. As a result, independent studies have suggested that most thymic squamous cell carcinomas, the most prevalent subtype (approximately 80%) of thymic carcinoma, exhibit features of medullary thymic epithelial cells (mTECs) (16-22).
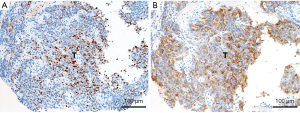
As a part of these studies, our group has demonstrated that, compared to thymomas, thymic carcinomas significantly highly express genes/proteins related to tuft cells, which are unique epithelial cells involving type 2 immunity and that were recently found to exist in the thymus as a subset of mTECs (23,24). Immunohistochemically, POU2F3, the master regulator of tuft cells (25), is significantly more expressed in thymic carcinoma (Figure 4A) than in thymomas, including epithelial rich, type B3 thymoma (16,17,21). POU2F3 expression is highly correlated with that of KIT proto-oncogene, receptor tyrosine kinase (KIT) (Figure 4B), a representative marker of thymic carcinomas (6,16). Other researchers have reported the expression of insulinoma-associated-1 (INSM1) (a new marker for neuroendocrine neoplasms) (22) and autoimmune regulator (AIRE), the most representative mTEC-related molecule (19,20) in thymic carcinoma. These findings could represent a paradigm shift in the histogenesis of thymic carcinomas, considering that they were historically regarded as tumors lacking the physiological properties of TECs. We applaud the renewed trend toward focusing on the histogenesis of TETs (26,27), and it may have value for diagnostic purposes as well (21). However, careful interpretations will be needed when inconsistent results are obtained among different studies. In addition, functional studies on the medullary phenotypes of thymic squamous cell carcinoma have not been reported; these studies must be conducted to make use of this feature in treatment decisions.
Possibility of more personalized medicine for thymic epithelial tumors
Because morphology reflects function, it is reasonable to assume that pathologically separated subtypes of thymomas and thymic carcinomas (6) have some biological differences. As mentioned, integrative unsupervised clustering with five different biological databases has divided TETs into four clusters (11). Although the current treatment guidelines simply look at whether a tumor is a thymoma or thymic carcinoma in order to determine treatment, it would not be ideal for personalized medicine based on the biological features of TETs.
As mentioned, the most important discovery in TET genetics is that of the GTF2I L424H mutation in most type A and AB thymomas (15). Recently, two groups have established mouse models of thymomas with mutations corresponding to human GTF2I L424H (28,29). With these tools, we may conduct in vivo functional analyses to investigate the significance of GTF2I in thymic epithelial cells and thymomas. The mutant GTF2I has already been found to cause different metabolic statuses in cultured cells in vitro (30). Thus, future studies may uncover the effect of GTF2I on drug metabolism and lead to optimal drug therapies for thymomas with the GTF2I L424H mutation.
Conclusions
Details of the 9th edition of the TNM classification system are as yet unknown, but the team responsible was established shortly after the launch of the 8th edition, and the upcoming edition has received continuous effort (10). The 9th edition will be based on more detailed and extensive data, and it surely address unresolved issues in the previous edition. Currently, the Masaoka-Koga system has been used alongside the TNM-8, and there is no question about its historical contribution. However, using two different (at least slightly) staging systems in the long term is not an ideal situation in that it hinders clear communication among researchers and clinicians and could lead to misunderstandings. Thus, all stakeholders working with TETs, including clinicians, radiologists, pathologists, and researchers, should work cooperatively across countries toward future editions of the TNM system being rationally accepted as the single staging system in use.
Acknowledgments
Funding: This work was supported by JSPS KAKENHI (No. JP21K06902 to Yosuke Yamada).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Masatsugu Hamaji) for the series “Locally Advanced Thymic Epithelial Tumors” published in Mediastinum. The article has undergone external peer review.
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-23-28/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-23-28/coif). The series “Locally Advanced Thymic Epithelial Tumors” was commissioned by the editorial office without any funding or sponsorship. Y.Y. receives a grant from JSPS KAKENHI (No. JP21K06902). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [Crossref] [PubMed]
- Koga K, Matsuno Y, Noguchi M, et al. A review of 79 thymomas: modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int 1994;44:359-67. [Crossref] [PubMed]
- Detterbeck FC, Nicholson AG, Kondo K, et al. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. J Thorac Oncol 2011;6:S1710-6. [Crossref] [PubMed]
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S65-72. [Crossref] [PubMed]
- Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours (8th ed). Oxford: Wiley-Blackwell; 2017.
- WHO Classification of Tumours Editorial Board. Thoracic tumours. International Agency for Research on Cancer; 2021. Available online: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours
- Ruffini E, Fang W, Guerrera F, et al. The International Association for the Study of Lung Cancer Thymic Tumors Staging Project: The Impact of the Eighth Edition of the Union for International Cancer Control and American Joint Committee on Cancer TNM Stage Classification of Thymic Tumors. J Thorac Oncol 2020;15:436-47.
- Kondo K, Van Schil P, Detterbeck FC, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the N and M components for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S81-7. [Crossref] [PubMed]
- Nicholson AG, Detterbeck FC, Marino M, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposals for the T Component for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S73-80. [Crossref] [PubMed]
- Ruffini E, Rami-Porta R, Huang J, et al. The International Association for the Study of Lung Cancer Thymic Epithelial Tumor Staging Project: Unresolved Issues to be Addressed for the Next Ninth Edition of the TNM Classification of Malignant Tumors. J Thorac Oncol 2022;17:838-51.
- Radovich M, Pickering CR, Felau I, et al. The Integrated Genomic Landscape of Thymic Epithelial Tumors. Cancer Cell 2018;33:244-258.e10. [Crossref] [PubMed]
- Nakamura S, Tateyama H, Nakanishi K, et al. Pleural Invasion Depth of Disseminated Nodules in Patients with Stage IVa or Recurrent Thymoma: Assessment, Curative Impact, and Surgical Outcomes. Ann Surg Oncol 2022;29:1829-37. [Crossref] [PubMed]
- Jasper K, Stiles B, McDonald F, et al. Practical Management of Oligometastatic Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:635-41. [Crossref] [PubMed]
- Suster DI, Craig Mackinnon A, DiStasio M, et al. Atypical thymomas with squamoid and spindle cell features: clinicopathologic, immunohistochemical and molecular genetic study of 120 cases with long-term follow-up. Mod Pathol 2022;35:875-94. [Crossref] [PubMed]
- Petrini I, Meltzer PS, Kim IK, et al. A specific missense mutation in GTF2I occurs at high frequency in thymic epithelial tumors. Nat Genet 2014;46:844-9. [Crossref] [PubMed]
- Yamada Y, Simon-Keller K, Belharazem-Vitacolonnna D, et al. A Tuft Cell-Like Signature Is Highly Prevalent in Thymic Squamous Cell Carcinoma and Delineates New Molecular Subsets Among the Major Lung Cancer Histotypes. J Thorac Oncol 2021;16:1003-16. [Crossref] [PubMed]
- Yamada Y, Sugimoto A, Hoki M, et al. POU2F3 beyond thymic carcinomas: expression across the spectrum of thymomas hints to medullary differentiation in type A thymoma. Virchows Arch 2022;480:843-51. [Crossref] [PubMed]
- Yuan X, Huang L, Luo W, et al. Diagnostic and Prognostic Significances of SOX9 in Thymic Epithelial Tumor. Front Oncol 2021;11:708735. [Crossref] [PubMed]
- Matsumoto M, Ohmura T, Hanibuchi Y, et al. AIRE illuminates the feature of medullary thymic epithelial cells in thymic carcinoma. Cancer Med 2023;12:9843-8. [Crossref] [PubMed]
- Li H, Ren B, Yu S, et al. The clinicopathological significance of thymic epithelial markers expression in thymoma and thymic carcinoma. BMC Cancer 2023;23:161. [Crossref] [PubMed]
- Naso JR, Vrana JA, Koepplin JW, et al. EZH2 and POU2F3 Can Aid in the Distinction of Thymic Carcinoma from Thymoma. Cancers (Basel) 2023;15:2274. [Crossref] [PubMed]
- Kashima J, Hashimoto T, Yoshida A, et al. Insulinoma-associated-1 (INSM1) expression in thymic squamous cell carcinoma. Virchows Arch 2022;481:893-901. [Crossref] [PubMed]
- Bornstein C, Nevo S, Giladi A, et al. Single-cell mapping of the thymic stroma identifies IL-25-producing tuft epithelial cells. Nature 2018;559:622-6. [Crossref] [PubMed]
- Miller CN, Proekt I, von Moltke J, et al. Thymic tuft cells promote an IL-4-enriched medulla and shape thymocyte development. Nature 2018;559:627-31. [Crossref] [PubMed]
- Yamashita J, Ohmoto M, Yamaguchi T, et al. Skn-1a/Pou2f3 functions as a master regulator to generate Trpm5-expressing chemosensory cells in mice. PLoS One 2017;12:e0189340. [Crossref] [PubMed]
- Yamada Y, Tomaru U, Ishizu A, et al. Expression of proteasome subunit β5t in thymic epithelial tumors. Am J Surg Pathol 2011;35:1296-304. [Crossref] [PubMed]
- Ströbel P, Hartmann E, Rosenwald A, et al. Corticomedullary differentiation and maturational arrest in thymomas. Histopathology 2014;64:557-66. [Crossref] [PubMed]
- Giorgetti OB, Nusser A, Boehm T. Human thymoma-associated mutation of the GTF2I transcription factor impairs thymic epithelial progenitor differentiation in mice. Commun Biol 2022;5:1037. [Crossref] [PubMed]
- He Y, Kim IK, Bian J, et al. A Knock-In Mouse Model of Thymoma With the GTF2I L424H Mutation. J Thorac Oncol 2022;17:1375-86. [Crossref] [PubMed]
- Kim IK, Rao G, Zhao X, et al. Mutant GTF2I induces cell transformation and metabolic alterations in thymic epithelial cells. Cell Death Differ 2020;27:2263-79. [Crossref] [PubMed]
Cite this article as: Yamada Y, Haga H. Pathological snapshots of thymic epithelial tumors with invasion into neighboring structures: preparing for the forthcoming revision of the TNM classification. Mediastinum 2023;7:36.


