
Diagnostic approach to prevascular (anterior) mediastinal lymphomas: when thoracic pathology meets hematopathology
Introduction
Lymphomas are among the most common malignant tumors occurring in the mediastinum, with the prevascular/anterior compartment being the most frequently affected site (1). Lymphomas at this location present as a mass with extension into adjacent structures and variable symptomatology including respiratory distress due to compression of the lower airways to superior vena cava syndrome in severe cases (2). Their incidence and differential diagnoses are strongly related to age and gender.
With less invasive tissue sampling techniques available today, such as mediastinoscopy, video assisted thoracoscopy video-assisted thoracic surgery (VATS), and imaging-guided biopsy, the mediastinal mass specimens evaluated by pathologists have shifted from large resections to fine needle aspirations and core biopsies. This imposes a diagnostic challenge not only due to sample size, but also because of the presence of artefacts. Despite best efforts to establish a diagnosis, not uncommonly small biopsies may offer limited information to further classify an anterior mediastinal lesion, and a recommendation to obtain an excisional biopsy should be discussed with the clinical and surgical teams. This is particularly important because the diagnosis of lymphoma carries significant therapeutic implications, such as the prompt initiation of chemotherapy and/or radiotherapy instead of a surgical resection that if delayed, may result in a detrimental prognostic impact to a patient.
Pathologists must be aware of the need to request sufficient material at the time of sampling. For some lymphomas establishing a diagnosis represents only the initial part of the work-up and anticipating the need to collect sufficient material for flow cytometry, cytogenetic and molecular studies is essential for prognostic and predictive purposes.
In this review, we focus on the practical diagnostic approach to the most common prevascular/anterior mediastinal lymphomas with an emphasis on the findings in small biopsies and provide tips on how to better triage these cases in surgical and thoracic pathology. In our experience, this practical diagnostic approach should start with specific questions that in general include: (I) is this a pediatric, a young adult, or an elderly patient? (II) Is this a primary anterior/prevascular mediastinal neoplasm, part of a systemic process with anterior/prevascular mediastinal involvement, or a metastasis? Following this approach, the path to a refined differential diagnosis and to a precise diagnosis should become easier or at least faster to request a hematopathology consultation for a more refined diagnosis.
Questions to be asked by pathologists when dealing with a prevascular/anterior mediastinal mass suspicious for lymphoma
In the pediatric population
Is this T-lymphoblastic lymphoma (T-LBL)? Is this normal thymus?
T-LBL commonly presents as an anterior mediastinal mass in children (3-5). Imaging typically shows a large mass with infiltration into the anterior chest wall, the great vessels, trachea, and lungs. The tumor can show cystic changes and hypodense areas that correspond to necrosis (2). Pleural effusions are relatively common. These features may also be seen with a “solid” small blue round cell tumor, but not in the normally large pediatric thymus that is lobulated and with well-demarcated borders.
If there is an anterior mediastinal mass suspicious for T-LBL, it is important to inquire if the patient has circulating T-lymphoblasts, lymphadenopathies, and/or cytopenias (suggestive of bone marrow involvement), which will favor a diagnosis of T-cell acute lymphoblastic leukemia (T-ALL/LBL) over that of normal thymus or a small blue round cell tumor. However, these features are not always present, and a biopsy of the mass may be performed to attempt to establish a diagnosis. Once the specimen is received, touch preparations should be made to determine if there is adequate material (never for diagnosis!) (Figure 1). If adequate, pathologists should communicate to the surgical team the need to obtain additional tissue, if possible, for flow cytometry, and for potential cytogenetic and molecular studies. Securing a sample for these purposes is crucial, since a subset of T-ALL/LBL present exclusively as an anterior mediastinal mass (no peripheral blood or bone marrow involvement) and the tissue from this mass may be the only one available to evaluate prognostic markers and potential targetable molecular alterations (6).
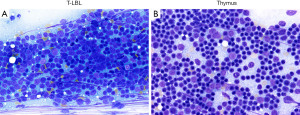
On histology, T-LBL presents as sheets of immature mononuclear cells with high nuclear to cytoplasmic ratio, moderately condensed chromatin, variable nucleolus, numerous mitoses, apoptotic cells, and necrosis with infiltration into the thymus and mediastinal soft tissues. A “starry-sky pattern” is commonly seen (Figure 2, left column). Conversely, biopsies composed predominantly of thymic cortex show somewhat similar morphology to T-LBL but without high grade cytologic features or increased proliferation, but this may be difficult to evaluate in a small or crushed specimen. The presence of well-defined borders from the surrounding fat or other structures, a dual population of epithelial cells and lymphocytes, and the lack of apoptosis and necrosis are typical features of normal thymic cortex (Figure 2, right column).

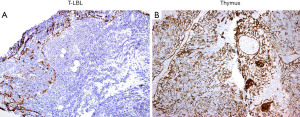
If concurrent flow cytometry was performed, detection of an aberrant immature T-cell population confirms T-LBL over normal thymus. If this is the case, a limited panel of immunohistochemical (IHC) markers could be performed but is not mandatory. On the other hand, if flow cytometry was not performed, IHC supports a diagnosis of T-LBL by confirmation of a hematopoietic origin by CD43+ or CD45+/−, T-cell differentiation (CD1a, CD3, CD2, CD5, CD7, CD4, CD8), expression of immature markers (TdT, CD34, CD10) and lack of myeloperoxidase as well as of the B-cell antigens CD19, CD20 and PAX5. Importantly, up to 20–30% of cases of T-LBL can be negative for either TdT, CD10, or CD34 and may show partial expression of CD79a (7). By IHC, the normal thymic cortex is also CD43+, CD45+, TdT+, and T-cell markers+, with characteristic CD1a+, CD4+/CD8+ (double positive), CD10−, and CD34−. Expression of CD10, CD34, or a CD4+/CD8−, CD4−/CD8+, CD4−/CD8− phenotype is always abnormal and supports T-LBL. Given the overlap in expression of some of these IHC markers, we recommend including pan-cytokeratin (pan-CK) which is extremely helpful to differentiate normal cortical thymus from T-LBL. Pan-CK highlights the epithelial network of the normal thymic cortex, whereas in T-LBL there is partial or complete disruption of this epithelial network (8) (Figure 3). Ki-67 is not useful since both T-LBL and the normal thymic cortex are highly proliferative. If limited tissue is available a minimal panel of IHC stains to distinguish between these conditions can include pan-CK, CD3 or CD7, PAX5, CD4, CD8, CD10, and CD34. TdT and CD1a may be reserved for a second round of IHC depending on the obtained results. A negative TdT and CD1a strongly supports T-LBL over thymus cortex because the former can be negative for these markers (7), but the latter is always TdT+ and CD1a+. However, if Hassall corpuscles are seen, then a negative TdT and CD1a should be interpreted with caution since these markers are negative in the thymic medulla.
Is this T-LBL? Is this a primary or metastatic small blue round cell tumor?
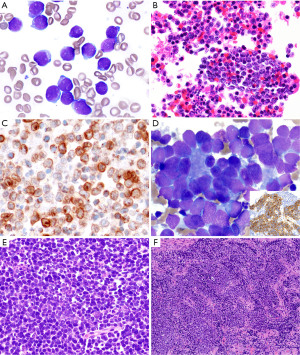
By morphology, the distinction between T-LBL and a small blue round cell tumor involving the anterior mediastinum can be challenging, but fortunately, a proper review of the clinical history and a limited panel of IHC stains is sufficient to exclude a hematopoietic tumor. Small blue round cell tumors are CD45−, CD43−, CD3−, PAX5−, myeloperoxidase− (Figure 4A-4C). Small blue round cell tumors that should be considered within the differential diagnosis of T-LBL in the pediatric population include metastases from neuroblastoma (synaptophysin+, chromogranin+, CD56/CD57+, NSE+, GFAP+, ALK+/−, CD99−, epithelial and myogenic markers−), retinoblastoma (synaptophysin+, chromogranin+, CD56/CD57+, NSE+, GFAP−, CD99−, epithelial and myogenic markers−), Ewing sarcoma and other primitive neuroectodermal tumors (CD99+, NKX2.2+, vimentin+, FLI1+, ERG+, cytokeratin+/−, myogenic markers−), and embryonal rhabdomyosarcoma (MyoD1+, desmin+) (Figure 4D-4F). T-LBL is positive for vimentin and CD99 and therefore, these markers do not definitively distinguish T-LBL from most small blue round cell tumors. Finally, an unusual case of pediatric nuclear protein in testis (NUT) carcinoma may also enter the differential diagnosis of T-LBL, especially when a small biopsy only contains the small cell component and not areas of squamous differentiation. Moreover, NUT carcinoma can be CD34+, CD45+/−, CD99+, and may lack expression of cytokeratins and p63 that may erroneously point to the diagnosis of a hematolymphoid tumor (9). Fortunately, NUT carcinoma does not express T-cell markers. Only a high index of suspicion for this neoplasm will trigger consideration to perform IHC for the NUT protein that will confirm or exclude this diagnosis.
The main features discussed in this section are summarized in Table 1.
Table 1
| Features | T-LBL | Normal thymus (cortex) | Metastatic small blue round cell tumors |
|---|---|---|---|
| Clinical | • May present with circulating blasts or cytopenias | • No other abnormalities | • No blasts, may produce cytopenias if bone marrow is involved |
| • SVC syndrome | • No SVC syndrome | • May or may not produce SVC syndrome | |
| • Check prior clinical history for malignancy elsewhere | |||
| Imaging | • Infiltrative mass, with or without pleural effusion | • Well-circumscribed, lobulated mass | • Infiltrative mass |
| • Metastasis may be present also at other sites | |||
| Morphology | • Sheets of immature cells with high N:C ratio, mitoses, apoptosis | • Two population of cells: epithelial and lymphoid | • Sheets of immature cells with high N:C ratio, mitoses, apoptosis |
| • Effacement of architecture | • No atypia, increased mitoses, or apoptosis | • Effacement of architecture | |
| • Widely infiltrative | • Well-preserved architecture with lobulated borders | • Widely infiltrative | |
| IHC | • Lymphoblasts: variable expression of T-cell markers, frequently CD3+, CD7+, may be CD4+/CD8+, CD4+/CD8−, CD4−/CD8+ or CD4−/CD8−, TdT+/−, CD1a+/−, CD45+/−, CD10+/−, CD34+/− | • Cortical thymocytes: T-cell markers+, CD1a+, CD4+/CD8+ (double positive), TdT+, ↑ Ki-67, CD10−, CD34− | • Variable immunophenotype depending on subtype (see text) |
| • Thymic epithelium: pan-CK disrupted pattern | • Thymic epithelium: pan-CK+ with reticular pattern, “nurse” cells can be seen | • All CD45−, T-cell markers− | |
| • Thymic epithelial cells: pan-CK disrupted pattern (if involving thymus) | |||
| • Pan-CK is positive in some tumors: NUT carcinoma | |||
| Other | • The mediastinal biopsy may be the only available material for prognostic and predictive testing (cytogenetics, molecular). Encourage collection of additional material for this purpose | • Thymus is normally cellular in children, do not call hyperplasia | • Some small blue round cell tumors are CD99+, not useful to distinguish from T-LBL |
| • Increased apoptotic cells may be seen in patients who have received steroids | • Rarely, NUT carcinoma may be pan-CK−, CD34+, CD45+/−, consider NUT IHC to exclude this diagnosis once other neoplasms have been excluded |
T-LBL, T-lymphoblastic lymphoma; SVC, superior vena cava; N:C, nuclear to cytoplasmic; pan-CK, pan-cytokeratin; NUT, nuclear protein in testis; IHC, immunohistochemistry.
Typically young adults
Is this nodular sclerosis classic Hodgkin lymphoma (NS-CHL), primary mediastinal (thymic) large B-cell lymphoma (PM-LBCL), or mediastinal gray zone lymphoma (mGZL)?
Both NS-CHL and PM-LBCL occur more commonly in young females, and they have similar clinical presentation with chest pain, cough, and dyspnea (10). On imaging, however, PM-LBCL tends to show infiltration into adjacent structures and superior vena cava syndrome that are not typical features of NS-CHL unless there is bulky disease (tumor size >10 cm). NS-CHL also tends to present with associated cervical and/or axillary lymphadenopathy, which is not a common feature in PM-LBCL (2). Regardless of the clinico-radiologic presentation, the diagnosis must be established by histopathology, and this will have significant repercussion for the type of chemotherapy regimen needed (11).
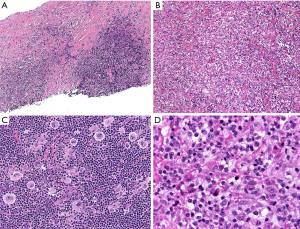
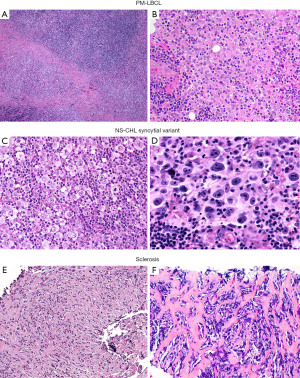
In typical cases, the morphologic distinction between these two lymphomas is readily established on hematoxylin and eosin stain. While NS-CHL consist of cellular nodules of a polymorphic infiltrate with variable number of Hodgkin/Reed-Sternberg (HRS) cells—usually of “lacunar” morphology—separated by fibrous bands, PM-LBCL presents as sheets of intermediate to large cells with oval to irregular nucleus, pale to clear eosinophilic cytoplasm and more delicate fibrosis with compartmentalization of the lymphoma cells into clusters, sometimes mimicking an infiltrating carcinoma (Figure 5). Granulomas are relatively frequent in NS-CHL but may be also seen in PM-LBCL. In these straightforward cases, IHC is only needed to support the suspected diagnosis by morphology (see below). Nevertheless, an issue arises with cases showing overlapping morphology, such as PM-LBCL with fibrous bands separating the tumor into cellular nodules (mimicking NS-CHL), or with the syncytial variant of NS-CHL showing sheets of HRS cells with variable necrosis (mimicking PM-LBCL) (Figure 6). Additional diagnostic issues include the presence of cellular distortion by crush artefact and of tumors with areas of NS-CHL and PM-LBCL or something “in between” (see below mGZL). For this kind of cases, IHC is mandatory to further classify the neoplastic process.
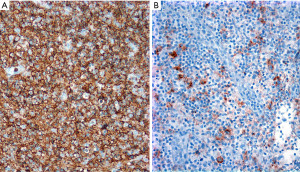
HRS cells are CD30+, CD15+ (60–70% of cases), weak PAX5+ when compared to background small B-cells, and MUM1+, while they are CD45−, CD20−, CD79a−, BOB.1−/+, OCT2−/+, and CD23− (12). CD30 is strong and with a Golgi and membranous or cytoplasmic pattern, while CD15 may be membranous, diffuse cytoplasmic, or granular. CD20 is usually negative or may be focal and weak in 20% of cases. The Epstein-Barr virus encoded RNA (EBER) by in situ hybridization is positive in ~20% of cases. The immunophenotype is the opposite in PM-LBCL, where the tumor cells are CD45+, CD20+, PAX5+ (strong), CD79a+, BOB.1+, OCT2+, CD30+ (variable/weak), MUM1+ (70%), p63 (70%), CD15−, and EBER− (13,14). Rather than performing all these IHC simultaneously and risk exhausting tissue, we recommend performing a limited panel of antibodies, namely CD3, CD20, PAX5, CD15 and CD30, that will confirm NS-CHL or PM-LBCL in most cases (Figures 7,8). If the morphology and IHC profile are not as described above, then further evaluation with CD23, CD45, other B-cell markers (CD79a, BOB.1, OCT2) and p63, may help to further support PM-LBCL or NS-CHL. At this point, if the morphology and IHC are discrepant, i.e., morphologic features of NS-CHL but with strong expression of B-cell markers, CD45+, weak or variable CD30, or PM-LBCL morphology but CD45−/+, B-cell markers−/+, CD30+, and CD15+, then the case is likely to represent mGZL (15,16). A hematopathology consultation should be done for these cases, which are challenging to classify given the subjectivity on what antibody to consider supportive or not of this diagnosis. mGZL is a very rare neoplasm with similar clinical and radiologic presentation as described for PM-LBCL and NS-CHL with the only difference that it occurs more frequently in men (17,18). However, the rarity of this neoplasm does not imply that most men with an anterior mediastinal mass will have a mGZL. This is always a diagnosis of exclusion.
The topography, morphology and immunophenotype of PM-LBCL is very characteristic and differs from that of conventional diffuse large B-cell lymphoma (DLBCL) in that it only rarely occurs in the anterior mediastinum (10). If a question arises about the subtype of a LBCL at this location, it is recommended to consult hematopathology for further subclassification. An important consideration related to PM-LBCL: An epithelioid neoplasm with clear cells in the anterior mediastinum with associated fibrosis and expression of p63 alone is not sufficient to diagnose non-keratinizing squamous cell carcinoma or thymic carcinoma with clear cell features. As mentioned above, ~70% of PM-LBCLs are positive for p63 (but not p40) and exclusion or confirmation of an hematolymphoid origin should be done with CD45, CD20/PAX5, and pan-CK (Figure 9). Expression of p63 may be useful to separate PM-LBCL from NS-CHL (19).
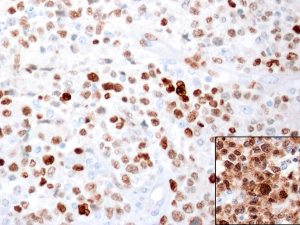
Rarely, cases of CD30+ anaplastic large B-cell lymphoma may lose expression of >1 B-cell marker (CD20, CD79a) and the use of additional B-cell markers (OCT2, BOB.1) may be required to further confirm a B-cell lineage.
Is this NS-CHL, PM-LBCL, or a mediastinal germ cell tumor?
Germ cell neoplasms are another anterior mediastinal tumor that should be considered in the differential diagnosis in young adults, either primary or metastatic to this location. If the history of a germ cell tumor from the testis or ovary is already known, the diagnosis should not pose difficulty. However, this may not be the case for primary mediastinal disease. If clinically suspected, the patient may already have results of serum tumor markers (beta-human chorionic gonadotropin, lactate dehydrogenase, alpha-fetoprotein) that will support germ cell tumor, but these studies may not be available at the time of initial biopsy.
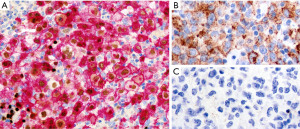
In a small biopsy, seminoma, embryonal carcinoma, and yolk sac tumor (solid or hepatoid variants) may resemble PM-LBCL since they are all composed of sheets of large, atypical cells, not uncommonly with clear cytoplasm (Figure 10). An obvious diagnostic component may not have been sampled or may be difficult to appreciate in crushed specimens. On the other hand, cases of a “burned out” germ cell tumor can mimic NS-CHL due to the presence of fibrosis, granulomatous inflammation, and variable number of large pleomorphic “HRS-like” cells (20) (Figure 11). Germ cell tumors involving an anterior mediastinal lymph node may also resemble lymphocyte-rich (LR) CHL secondary to the presence of large, atypical cells in a background of small lymphocytes. Fortunately, a limited panel of IHC stains helps to readily distinguish lymphomas from germ cell tumors. Seminoma and embryonal carcinoma are OCT3/4+ and SALL4+ and embryonal carcinoma is pan-CK+ and CD30+ (Figure 10B,10C). Yolk sac tumors are pan-CK+, alpha-fetoprotein+, SALL4+, glypican-3+, and OCT3/4−. Primary mediastinal seminomas are also frequently positive for pan-CK in contrast to those from gonadal origin. Germ cell tumors are CD45−, B-cell markers−, and CD15− which rules out NS-CHL and PM-LBCL. Unusual positivity for beta-human chorionic gonadotropin or alpha-fetoprotein has been reported in NS-CHL and/or PM-LBCL, which could erroneously point to a diagnosis of germ cell tumor with choriocarcinoma or yolk sac tumor components (21,22). Again, the expression of lymphoid markers, lack of pan-CK and other germ cell markers readily solves this issue. Only extremely rarely do lymphomas and germinomas coincide, which can be a consideration when separate components are seen in the same specimen.

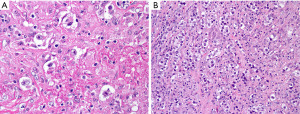
Is this PM-LBCL or B3 (atypical) thymoma?
Clinically, B3 thymoma occurs in individuals older than the expected age for PM-LBCL, but there is some overlap in the age of presentation (23). World Health Organization classification (WHO) B3 thymoma (atypical thymoma in the Suster-Moran classification) (24) is a neoplasm composed of sheets of polygonal epithelial cells with random atypia, oval nucleus with variable prominent nucleolus, and abundant pale eosinophilic cytoplasm with well-defined borders (Figure 12). This tumor is usually easy to recognize as an epithelial neoplasm but in small specimens it may sometimes resemble large cell lymphoma, namely PM-LBCL. Morphologic clues to support thymoma are the presence of small lymphocytes around capillaries (perivascular spaces) and focal “squamoid” features. IHC for pan-CK and CD45 is the easiest initial step to confirm if a lesion with sheets of polygonal cells is lymphoma (CD45+, pan-CK−) or thymoma (CD45−, pan-CK+). Once the lineage has been established, additional markers can be performed to characterize the process as thymoma (p40+, polyclonal PAX8+) or PM-LBCL (CD20 or PAX5+, CD30+/−, MUM1+, CD23+).
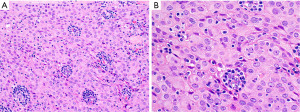
The main features discussed in this section are summarized in Table 2.
Table 2
| Features | PM-LBCL | NS-CHL | M-GZL | Germ cell tumor | WHO B3 thymoma (atypical thymoma—S&M) |
|---|---|---|---|---|---|
| Clinical | • Young F > M | • Young F > M | • M > F | • No gender preference | • Slight male predominance |
| • May present with SVC syndrome | • No SVC syndrome unless mass >10 cm (bulky disease) | • May present with SVC syndrome | • Usually, no SVC syndrome | ||
| • May be accompanied by cervical or axillary LAPD | |||||
| Imaging | • Infiltrative mass into adjacent structures | • Usually, well-circumscribed mass | • Infiltrative or well-circumscribed | • Usually, a very large mass with invasion or significant compression of adjacent structures | • Relatively well-circumscribed mass, but may show infiltrative borders |
| Morphology | • Sheets of clear cells with oval to irregular nucleus, variable fibrosis, may mimic infiltrating carcinoma | • Polymorphic infiltrate in nodules separated by fibrotic bands | • May show features of PM-LBCL, NS-CHL or a mixture | • Sheets of epithelioid cells w/wo clear cytoplasm mimicking LBCL | • Sheets of epithelioid cells, sometimes clear |
| • With scattered HRS cells | • ‘Burned out’ tumors with few tumor cells in fibrous tissue and granulomas mimicking CHL | • Perivascular lymphocytes | |||
| • Granulomas | • May show squamoid features and cysts | ||||
| IHC | • CD20+ | • HRS cells: CD20– | • Morphology of PM-LBCL with CHL immunophenotype or, morphology of NS-CHL with PM-LBCL immunophenotype | • Negative for hematolymphoid markers (CD45–, CD20–) | • Negative for hematolymphoid markers (CD45–, CD20–) |
| • PAX5+ strong | • PAX5+ dim | • Consult hematopathology | • Variable immunophenotype depending on subtype (see text) | • Pan-CK+, p63+, p40+ | |
| • CD30+/– | • CD30+ | • CD30+ but pan-CK+ in embryonal carcinoma | • pPAX8+ | ||
| • CD15– | • CD15+/– | ||||
| • CD45+ | • CD45– | ||||
| • CD23+ | • CD23– | ||||
| • MUM1+ | • MUM1+ | ||||
| • p63+ (70%) | • p63– | ||||
| • p40– | • pan-CK– | ||||
| • pan-CK– | • PAX8– | ||||
| • PAX8– |
PM-LBCL, primary mediastinal (thymic) large B-cell lymphoma; NS-CHL, nodular sclerosis classic Hodgkin lymphoma; M-GZL, mediastinal gray zone lymphoma; WHO, World Health Organization classification; S&M, Suster and Moran classification; F, female; M, male; SVC, superior vena cava; LAPD, lymphadenopathy; w/wo, with/without; HRS cells, Hodgkin/Reed-Sternberg cells; IHC, immunohistochemistry; pan-CK, pan-cytokeratin; pPAX8, polyclonal PAX8 antibody.
Typically older adults
Is this T-LBL, B1 thymoma, or true thymic hyperplasia?
T-ALL/LBL may also present as a prevascular/anterior mediastinal mass in older individuals (25). However, the differential diagnosis is entirely different from that of pediatric patients. On imaging, T-LBL typically presents a large mass with infiltration into adjacent structures, as described above (see section “Is this T-lymphoblastic lymphoma? Is this normal thymus?”). The tumor can show cystic changes and hypodense areas that correspond to necrosis. Pleural effusions are common. These features may also be seen with a poorly differentiated tumor involving/extending to this compartment (lung cancer, thymic neuroendocrine tumor/carcinoma) or a metastasis, but not in thymic hyperplasia or thymoma. These last two conditions can grow to a significant large size but typically demonstrate well-demarcated borders. Similar to what has been described for pediatric patients, in older adults it is also relevant to inquire if the patient has circulating T-lymphoblasts, lymphadenopathies, and/or cytopenias (suggestive of bone marrow involvement), which will favor a diagnosis of T-ALL/LBL over thymoma, thymic hyperplasia or a poorly differentiated solid tumor. Moreover, a prior history of malignancy elsewhere is helpful to favor metastasis over a primary mediastinal tumor, at least on first impression. We cannot stress enough that a subset of T-ALL/LBL presents as an anterior mediastinal mass without peripheral blood or bone marrow involvement and therefore, securing a sample to evaluate prognostic markers and potential targetable molecular alterations is crucial, since the material from the mediastinal mass may be the only one available for these purposes (6).
Morphologically, anterior mediastinal tumors that may pose a problem with T-LBL in a small biopsy include lymphocyte-rich thymoma (WHO B1) and true thymic hyperplasia, particularly when the specimen shows predominantly thymic cortex. The same diagnostic approach and histologic features described for T-LBL and true thymic hyperplasia (thymus not involuted as expected for age) listed above should be followed here (see section “Is this T-lymphoblastic lymphoma? Is this normal thymus?” and Figure 2).
In contrast to T-LBL, B1 thymoma has a dual population of epithelial cells and lymphocytes, without significant atypia or increased proliferation activity. The presence of perivascular spaces with small lymphocytes and medullary differentiation supports the diagnosis of thymoma over T-LBL. If the periphery of the tumor is seen, there may be an appreciation of lobulations and sharply demarcated borders from the mediastinal fat (Figure 13). The identification of Hassall corpuscles confirms the biopsy site as thymus but does help to distinguish thymoma from T-LBL. In a small biopsy, the diagnostic features of thymoma may not be easy to identify and there may be a paucity of epithelial cells making the histologic distinction with T-LBL challenging. IHC, flow cytometry and/or molecular studies may be of help to further clarify the diagnosis.
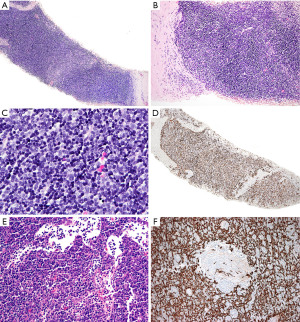
The IHC prolife of T-LBL and true thymic hyperplasia (thymus not involuted as expected for age) has been already described in the section “Is this T-lymphoblastic lymphoma? Is this normal thymus?”. The lymphoid component of B1 thymoma will be identical to that of the normal thymus since these cells are not neoplastic. Given the overlap of markers, we again recommend including pan-CK in the IHC panel that is extremely helpful to differentiate hyperplastic thymus and B1 thymoma from T-LBL (26). Pan-CK highlights the epithelial network of the hyperplastic thymic cortex and that of thymomas (“lace-like” pattern), whereas in T-LBL there is partial or complete disruption of this epithelial network (Figure 13D). Pan-CK also highlights the perivascular spaces of thymoma as “pan-CK- spaces”, a feature not seen in T-LBL (Figure 13F). In the same line, the polyclonal PAX8 antibody is also helpful to distinguish thymoma (PAX8+) from T-LBL (PAX8−) (26), but this is not the case with the PAX8 monoclonal antibody that is negative in thymoma (27). It is important to be aware of this difference to avoid a misinterpretation of this marker. A clue that the PAX8 antibody used is polyclonal is to look at the lymphocytes; if the B-cells are positive, then this is the polyclonal antibody (PAX8 cross reacts with PAX5 present in B-cells) (28). If limited tissue is available, a short panel of IHC stains to distinguish between these conditions can include pan-CK, CD3 or CD7, PAX5, CD4, CD8, CD10, and CD34. TdT and CD1a may be reserved for a second round of immunostains depending on the obtained results. A negative TdT and CD1a strongly supports T-LBL over thymus cortex because the former can be negative for these markers (7), but the latter is always TdT+ and CD1a+. However, if Hassall corpuscles are seen, then a negative TdT and CD1a should be interpreted with caution since these markers are negative in the thymic medulla.
If flow cytometry is available, T-LBL is typically composed of an aberrant immature T-cell population and B1 thymoma and normal thymus show T-cells at various stages of maturation. However, in some instances T-LBL may not show an aberrant immunophenotype by flow cytometry and distinction from normal thymocytes may not be possible. In this instance, close communication with a hematopathologist is needed as to consider the clinical scenario and decide to further perform TCR gene rearrangement to asses for the presence or absence of a clonal T-cell population.
Is this T-LBL, or a poorly differentiated tumor with small blue round cell morphology involving the anterior mediastinum?
By morphology, the distinction between T-LBL and a poorly differentiated malignant neoplasm with small cell morphology involving/extending to the anterior mediastinum can be challenging. Imaging is helpful to determine if the mass is a tumor extending from the lung (either a small or non-small cell carcinoma) or may be primary from the thymus (neuroendocrine tumor/carcinoma). Clinical history is useful to consider a metastasis if the patient has a prior diagnosis of malignancy elsewhere. A proper panel of IHC stains excludes a hematopoietic tumor (CD45−, CD43−, CD3−, PAX5−, myeloperoxidase−). Further IHC should be tailored in respect to the clinical and imaging findings, namely pan-CK, neuroendocrine markers (synaptophysin, chromogranin, INSM1), and TTF1 for lung cancer extending or metastatic to the prevascular mediastinum; S100, HMB-45, Melan-A, SOX10 for metastatic amelanotic melanoma; pan-CK, p40, neuroendocrine markers for thymic neuroendocrine tumor/carcinoma; PSA, PSAP or NKX3.1 for metastatic prostate cancer; CK7 and GATA3 for breast cancer, to name a few examples. The main features discussed in this section are summarized in Table 3.
Table 3
| Features | T-LBL | True thymic hyperplasia | WHO B1 thymoma (lymphocyte rich) | Metastatic small blue round cell tumor |
|---|---|---|---|---|
| Clinical | • May present with circulating blasts or cytopenias | • No other abnormalities | • Slight female predominance | • No blasts, may produce cytopenias if bone marrow is involved |
| • SVC syndrome | • No SVC syndrome | • Usually, no SVC syndrome | • May or may not produce SVC syndrome | |
| • Check prior clinical history for malignancy elsewhere | ||||
| Imaging | • Infiltrative mass, with or without pleural effusion | • Well-circumscribed, lobulated mass | • Relatively well-circumscribed mass, but may show infiltrative borders | • Infiltrative mass |
| • May show extension from adjacent structures (lung, other) | ||||
| • Metastasis may be present also at other sites | ||||
| Morphology | • Sheets of immature cells with high N:C ratio, mitoses, apoptosis | • Two population of cells: epithelial and lymphoid | • Two population of cells: lymphoid > epithelial | • Sheets of immature cells with high N:C ratio, mitoses, apoptosis |
| • Effacement of architecture | • No atypia, increased mitoses, or apoptosis | • No atypia, increased mitoses, or apoptosis | • Effacement of architecture | |
| • Widely infiltrative | • Well-preserved architecture with lobulated borders | • Well-preserved architecture with lobulated borders | • Widely infiltrative | |
| • Medullary differentiation | ||||
| • Perivascular spaces | ||||
| IHC | • Lymphoblasts: variable expression of T-cell markers, frequently CD3+, CD7+, may be CD4+/CD8+, CD4+/CD8−, CD4−/CD8+ or CD4−/CD8−, TdT+/−, CD1a+/−, CD45+/−, CD10+/−, CD34+/−, PAX8− | • Cortical thymocytes: T-cell markers+, CD1a+, CD4+/CD8+ (double positive), TdT+, ↑ Ki-67, CD10−, CD34− | • Cortical thymocytes: T-cell markers+, CD1a+, CD4+/CD8+ (double positive), TdT+, ↑ Ki-67, CD10−, CD34− | • Variable immunophenotype depending on subtype (see text) |
| • Thymic epithelium: pan-CK+ with reticular pattern, “nurse” cells can be seen, pPAX8+ | • Thymic epithelium: pan-CK+ with reticular pattern, pPAX8+ | • All CD45−, T-cell markers− | ||
| • Thymic epithelium: pan-CK disrupted pattern | • Thymic epithelial cells: pan-CK disrupted pattern (if involving thymus) | |||
| • Pan-CK+ in poorly differentiated carcinomas, including thymic neuroendocrine carcinoma, and NUT carcinoma | ||||
| • PAX8+ consider metastasis from thyroid, genitourinary or gynecologic tract | ||||
| Other | • The mediastinal biopsy may be the only available material for prognostic and predictive testing (cytogenetics, molecular). Encourage collection of additional material for this purpose | • Normal thymus histology but not appropriate for age (no involution) | • Increased apoptotic cells may be seen in patients who have received steroids | • Rarely, NUT carcinoma may be pan-CK−, CD34+, CD45+/−, consider NUT IHC once other neoplasms have been excluded |
| • May develop after chemotherapy is stopped in CHL (“rebound hyperplasia”) | • Preferred to sign out a small biopsy as “thymic tissue, differential diagnosis includes thymic hyperplasia and B1 thymoma” | |||
| • Preferred to sign out a small biopsy as “thymic tissue, differential diagnosis includes thymic hyperplasia and B1 thymoma” | • Although rare in children, the most common type of thymic epithelial neoplasm in this group |
T-LBL, T-lymphoblastic lymphoma; WHO, World Health Organization classification; SVC, superior vena cava; N:C, nuclear to cytoplasmic; IHC, immunohistochemistry; pan-CK, pan-cytokeratin; pPAX8, polyclonal PAX8 antibody; NUT, nuclear protein in testes; CHL, classic Hodgkin lymphoma.
Is this B2 thymoma, LR-CHL, or metastatic nasopharyngeal carcinoma?
WHO B2 thymoma contains about the same proportion of epithelial cells and lymphocytes as compared to B1 thymoma. This tumor may pose a differential diagnosis with LR-CHL (Figure 14) or with metastatic nasopharyngeal carcinoma to the anterior mediastinum, both of which, however, are extremely uncommon tumors at this location. IHC for pan-CK, p40, CD15, CD30, PAX5, and EBER in situ hybridization are helpful to distinguish between these three entities. The epithelial cells in B2 thymoma do not show significant atypia and are pan-CK+, p40+, CD15−, CD30−, PAX5−, EBER− and the background is composed of thymocytes (TdT+, CD1a+, CD4+/CD8+). In LR-CHL, the HRS cells are atypical and more pleomorphic and pan-CK−, p40−, CD15+/−, CD30+, PAX5+ (dim), EBER+, and the background is usually B-cell predominant. Nasopharyngeal carcinoma shows significant more atypia than B2 thymoma and is pan-CK+, p40+, CD15−, CD30−, PAX5−, but EBER+, with a mixed background of mature T-cells and B-cells, if metastatic to a lymph node. The main features discussed in this section are summarized in Table 4.
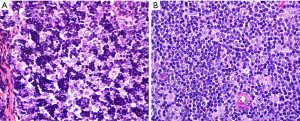
Table 4
| Features | WHO B2 thymoma | Lymphocyte-rich CHL | Metastatic nasopharyngeal carcinoma |
|---|---|---|---|
| Clinical | • No gender predilection | • Extremely rare at this location | • M > F |
| • Usually, no SVC syndrome | • More prevalent in Asian population | ||
| Imaging | • Relatively well-circumscribed mass, but may show infiltrative borders | • Anterior mediastinal mass/lymphadenopathy | • Anterior mediastinal mass/lymphadenopathy |
| Morphology | • Two population of cells: lymphoid = epithelial | • Scattered large, atypical cells (HRS cells) in a lymphocyte-rich background, epithelioid macrophages in variable numbers | • Scattered large, atypical epithelioid cells in a lymphocyte-rich background (may be a lymph node, or thymus) |
| • No atypia, increased mitoses, or apoptosis | • May or may not show squamous features | ||
| • Well-preserved architecture with lobulated borders | |||
| • Medullary differentiation | |||
| • Perivascular spaces | |||
| IHC | • Cortical thymocytes: T-cell markers+, CD1a+, CD4+/CD8+ (double positive), TdT+, ↑ Ki-67, CD10−, CD34− | • HRS cells: CD20− | • All hematolymphoid markers negative (CD45−, T-cell and B-cell markers−) |
| • Thymic epithelium: pan-CK+ with reticular pattern, pPAX8+ | • PAX5+ dim | • CD30− | |
| • CD30+ | • CD15− | ||
| • CD15+/− | • EBER+ | ||
| • CD45− | • pan-CK+, PAX8− | ||
| • MUM1+ | • p63+, p40+ | ||
| • EBER+ | |||
| • pan-CK−, PAX8− | |||
| • p63−, p40− | |||
| Other | • Increased apoptotic cells may be seen in patients who have received steroids | • If present, likely to be a known history of widespread disease | • Anterior mediastinum rare site of metastasis |
WHO, World Health Organization classification; CHL, classic Hodgkin lymphoma; M, male; F, female; SVC, superior vena cava; HRS cells, Hodgkin/Reed-Sternberg cells; IHC, immunohistochemistry; pan-CK, pan-cytokeratin; pPAX8, polyclonal PAX8 antibody.
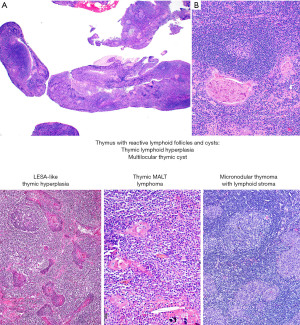
Is this thymic marginal zone lymphoma, lymphoepithelial-sialadenitis-like thymic hyperplasia, thymic lymphoid hyperplasia, a multilocular thymic cyst (MTC), or micronodular thymoma with lymphoid stroma (MTLS)?
Thymic marginal zone lymphoma of mucosa-associated lymphoid tissue (thymic MALT lymphoma) occurs in older individuals (median age 63 years) and many patients have an underlying autoimmune disease, such as Sjögren syndrome or rheumatoid arthritis (29-36). In contrast, thymic lymphoid hyperplasia and MTC occur in a younger population, but there may be some overlap with thymic MALT lymphoma in the age of presentation. One should keep in mind that thymic MALT lymphoma and its likely precursor, lymphoepithelial sialadenitis (LESA)-like thymic hyperplasia, are very rare diseases (37,38). Therefore, one is more likely to encounter thymic lymphoid hyperplasia or MTC than these other two conditions. MTLS, a rare subtype of thymoma, also enters the differential diagnosis of the entities discussed here because of the presence of abundant B-cells and lymphoid follicles (26). MTLS and thymic lymphoid hyperplasia may be associated with myasthenia gravis and less commonly with other autoimmune disorders.
On imaging, thymic MALT lymphoma, thymic hyperplasia, MTC and MTLS present as a relatively well-defined anterior mediastinal mass with solid and cystic components (39,40). Some patients with thymic MALT lymphoma may also have pulmonary cysts or nodular pulmonary amyloidosis that may or may not be related to Sjögren syndrome (32). Given the overlap on imaging, histopathologic evaluation is required to establish a definitive diagnosis. This is crucial because the treatment for thymic MALT lymphoma (confined to the gland) (33), MTC or MTLS consist of surgical resection, whereas for thymic lymphoid hyperplasia surgery may or may not be required (i.e., myasthenia gravis refractory to therapy).
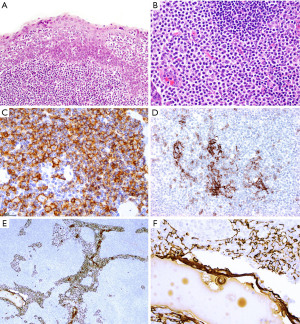
Microscopically, all these conditions share the presence of a brisk lymphoid inflammatory infiltrate with variable number of reactive follicles in the thymus parenchyma and variable number of epithelial cysts that may contain proteinaceous fluid and/or cholesterol granulomas (Figure 15). The cysts may be lined by squamous epithelium and sometimes by columnar or respiratory epithelium. Additional morphologic features and IHC results are helpful to further distinguishing them. Thymic MALT lymphoma is a process that effaces the thymic architecture. It is composed of sheets of small lymphocytes with scattered large immunoblast-like cells, variable number of monocytoid cells and reactive lymphoid follicles with prominent marginal zones. The marginal zone lymphocytes infiltrate and distort the germinal centers, a feature called “follicular colonization”. In addition, there are conspicuous lymphoepithelial lesions (intraepithelial lymphocytes within cyst epithelium or within Hassall corpuscles), and monocytoid cells surrounding Hassall corpuscles. Plasma cells are present in variable number and distribution and may or may not have Dutcher bodies. Conversely, thymic lymphoid hyperplasia, LESA-like thymic hyperplasia and MTC distort but do not efface the thymus architecture, and there are no sheets of monocytoid cells, expanded marginal zones, “follicular colonization”, or conspicuous lymphoepithelial lesions. Plasma cells are present but are not increased. In addition, in MTC the cystic component is prominent, hence the name. In LESA-like thymic hyperplasia there is proliferation of thymic epithelium and Hassall corpuscles that militates against the diagnosis of thymic MALT lymphoma (37). MTLS has similar features as described for thymic lymphoid hyperplasia and MTC but in this lesion the epithelial component corresponds to the neoplastic process and is seen as islands of thymic epithelium with variable shapes in between reactive lymphoid follicles.
IHC is of aid to distinguish between these thymic lymphoid lesions (Figures 16,17). In thymic MALT lymphoma most of the small lymphocytes, including the monocytoid cells, are CD20+, PAX5+, CD79a+, bcl-2+, and negative for CD5, CD10, bcl-6, and cyclin D1. CD43 aberrant co-expression in B-cells is seen in 40–50% of cases. CD3 highlights variable amount of background small T-cells and residual thymocytes. The germinal center markers CD10, and bcl-6 highlight normal and distorted germinal centers (“follicular colonization”), whereas the follicular dendritic cell (FDC) markers CD21 or CD23 highlight disrupted follicular dendritic cell meshworks (FDCM). Plasma cells and cases with plasmacytic differentiation are CD138+, usually IgA+, and may or may not show kappa or lambda light chain restriction. The proliferation index by Ki-67 is low (<30%) in the neoplastic lymphocytes and high in residual germinal centers. Pan-CK is helpful to assess the preservation of the thymic architecture that may not be obvious on routine stains as well as to highlight the presence of lymphoepithelial lesions. The intraepithelial lymphocytes can also be highlighted with CD20 or PAX5 (Figure 16).
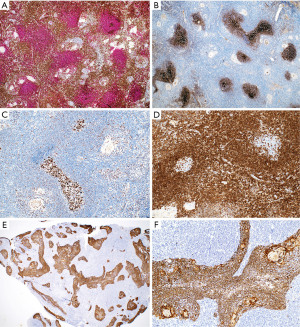
In contrast to the findings described above, in thymic lymphoid hyperplasia, LESA-like thymic hyperplasia and MTC, assessment of CD3 and CD20 shows a proper compartmentalization of B-cells in lymphoid follicles and of T-cells in interfollicular areas and there is a T-cell predominance. The germinal centers are negative for bcl-2 and there is no, or only occasional disruption of a lymphoid follicle appreciated with germinal center markers or FDC markers (Figure 17). CD43 highlights T-cells and is negative in B-cells. The plasma cells are always polytypic. Pan-CK highlights the epithelial lining of cysts that is sometimes attenuated, as well as residual Hassall corpuscles, but no significant number of intraepithelial lymphocytes are seen, or this are mostly CD3+ T-cells rather than CD20+ B-cells. Pan-CK is helpful to highlight the neoplastic epithelial cells in MTLS, showing clusters of epithelial cells in between follicles, which is not a feature of the processes described above (Figure 18). The lymphoid component of MTLS is reactive as described for thymic lymphoid hyperplasia or MTC.
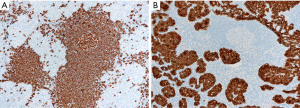
If the tissue is scant or limited, the distinction between a neoplastic or reactive thymic lymphoid lesion may be challenging given the significant morphologic overlap between all these processes. In this instance, we recommend giving a descriptive diagnosis of “thymic tissue with lymphoid hyperplasia and benign epithelial cysts” and give a differential diagnosis to include all the lesions described above. A hematopathology consultation will be helpful to at least attempt to exclude lymphoma. If available, flow cytometry is helpful to confirm or exclude MALT lymphoma if there is detection of a monotypic CD5−/CD10− B-cell population.
Moreover, close communication with the clinical team is crucial not only to discuss the next steps for these kind of cases (i.e., observation, new biopsy attempt with collection for flow cytometry or other ancillary studies, resection), but also to recommend a screening evaluation for an underlying autoimmune disorder if a patient does not have a known history of such a disorder. The main features discussed in this section are summarized in Table 5.
Table 5
| Features | Thymic MALT lymphoma | Thymic lymphoid hyperplasia | LESA-like thymic hyperplasia | Multilocular thymic cyst | Micronodular thymoma with lymphoid stroma |
|---|---|---|---|---|---|
| Clinical | • Median 63 years old | • Younger individuals | • No apparent age or gender predilection (very few cases reported) | • No age or gender predilection | • Slight male predominance |
| • May be asymptomatic or produce symptoms related to compression | • May be asymptomatic or produce symptoms related to compression | • May be asymptomatic or produce symptoms related to compression | • May be asymptomatic or produce symptoms related to compression | • May be asymptomatic or produce symptoms related to compression | |
| • No SVC syndrome | • No SVC syndrome | • No SVC syndrome | • No SVC syndrome | • No SVC syndrome | |
| • Association with non-MG disorders (SS, RA) | • Association with MG | • Association with non-MG disorders (SS, RA) | • No association with MG, SS or RA | • No association with MG, SS or RA | |
| • Very rare | • Very rare | ||||
| Imaging | • In general, a relatively well-defined anterior mediastinal mass with solid and cystic components | ||||
| Morphology | • Effacement of thymic architecture by small lymphocytes, monocytoid cells and few large cells | • Normal thymus with brisk lymphoid infiltrate with reactive LFs | • Epithelial and Hassall corpuscle hyperplasia with brisk lymphoid infiltrate with reactive LFs | • Multiple cysts and thymus with brisk lymphoid infiltrate with reactive LFs | • Epithelial proliferation (islands or coalescing areas) in between brisk lymphoid infiltrate with reactive LFs |
| • Variable number of plasma cells | • Few plasma cells | • Prominent LELs | • Disruption but not complete effacement of thymic architecture | • No LELs | |
| • Reactive LFs with expanded MZ cells and “follicular colonization” | • Disruption but not complete effacement of thymic architecture | • No monocytoid cells | • No or rare LELs | • No monocytoid cells | |
| • LELs | • No or rare LELs | • Few plasma cells | • No monocytoid cells | • No expanded MZ or follicular colonization | |
| • Variable cyst formation | • No monocytoid cells | • Disruption but not complete effacement of thymic architecture | • Few plasma cells | • Few plasma cells | |
| • No expanded MZ or follicular colonization | • No expanded MZ or follicular colonization | • No expanded MZ or follicular colonization | • Variable cyst formation | ||
| • Variable cyst formation | • Variable cyst formation | ||||
| IHC | • Usually B-cells > T-cells | • T-cells > B-cells | • Usually T-cells > B-cells | • T-cells > B-cells | • T-cells > B-cells |
| • Lymphoma cells: CD20+, bcl-2+ | • T-cells: interfollicular | • T-cells: interfollicular | • T-cells: interfollicular | • T-cells: interfollicular | |
| • CD5−, CD10−, bcl-6−, cyclin D1− | • B-cells: LFs | • B-cells: LFs, may also form part of LELs | • B-cells: LFs | • B-cells: LFs | |
| • Ki-67 low | • LFs: CD10+, bcl-6+ | • LFs: CD10+, bcl-6+ | • LFs: CD10+, bcl-6+ | • LFs: CD10+, bcl-6+ | |
| • Residual LFs: CD10+, bcl-6+ | • bcl-2−, Ki-67 high | • bcl-2−, Ki-67 high | • bcl-2−, Ki-67 high | • bcl-2−, Ki-67 high | |
| • bcl-2-, Ki-67 high | • CD21, CD23 normal FDCM | • CD21, CD23 normal FDCM | • CD21, CD23 normal FDCM | • CD21, CD23 normal FDCM | |
| • CD21, CD23 disrupted FDCM | • Plasma cells: polytypic | • Plasma cells: polytypic | • Plasma cells: polytypic | • Plasma cells: polytypic | |
| • Plasma cells: may be polytypic or monotypic | • Pan-CK+ attenuated but not significantly disrupted epithelium and cysts | • Pan-CK+ hyperplastic epithelial elements and cysts | • Pan-CK+ attenuated but not significantly disrupted epithelium and cysts | • Pan-CK+ neoplastic epithelium, significant component of the tumor | |
| • Pan-CK+ in scant or effaced thymic epithelium, and cysts | |||||
| Other | • FC: monotypic B-cells and/or plasma cells | • FC: polytypic B-cells | • FC: polytypic B-cells | • FC: polytypic B-cells | • FC: polytypic B-cells |
| • Complete resection is usually curative; may need single-agent chemotherapy | • Most common of these lesions to be encountered | • Strong association with thymic MALT lymphoma | • Rarely associated with thymoma, carcinoma, or MALT lymphoma | • Rare variant of thymoma | |
MALT, marginal zone lymphoma of mucosa-associated lymphoid tissue; LESA, lymphoepithelial sialadenitis; SVC, superior vena cava; MG, myasthenia gravis; SS, Sjögren syndrome; RA, rheumatoid arthritis; MZ, marginal zone; LELs, lymphoepithelial lesions; LFs, lymphoid follicles; IHC, immunohistochemistry; FDCM, follicular dendritic cell meshworks; pan-CK, pan-cytokeratin; FC, flow cytometry.
The differential diagnosis between MALT lymphoma and other small B-cell lymphomas is not included here since practically other small B-cell lymphomas are exceedingly rare in the thymus.
Miscellaneous: is this hyaline-vascular Castleman disease (hv-CD) or lymphoma?
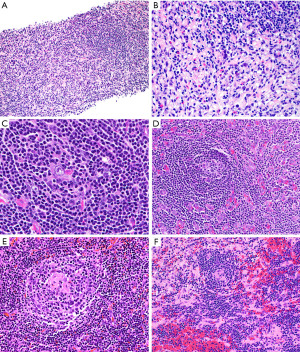
For purpose of this manuscript, we will only discuss hv-CD. This is a lymphoid disorder that not uncommonly occurs in the prevascular/anterior mediastinum. This variant almost always corresponds to unicentric CD (clinical presentation), with localized involvement and lack of systemic symptoms, generalized lymphadenopathies or organomegalies (41,42). On imaging, hv-CD presents as a relatively well-circumscribed mass that depending on the size may or may not cause compression of adjacent structures. Asymptomatic cases are usually detected incidentally on imaging performed for other reasons. When symptoms are present, they are related to mass effect produced towards adjacent structures, such as chest pain, cough, or shortness of breath (41,42). Superior vena cava syndrome is rare. Histopathologic evaluation is required to establish the diagnosis.
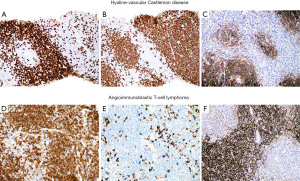
The typical features of hv-CD may or may not be seen in a small biopsy. If present, they include atrophic germinal centers with occasional dysplastic FDCs, multiple layers of mantle zone cells with concentric arrangement (“onion-skinning”), hyalinized blood vessels piercing into a germinal center (“lollipop lesions”) and interfollicular areas with increased vascularity and scattered dysplastic DCs (43). However, when these features are not obvious in a biopsy there is a potential for misinterpreting hv-CD as lymphoma (Figure 19). The presence of numerous follicles with “atypical cells” (dysplastic FDCs misinterpreted as centroblasts) may be confused with follicular lymphoma (44) or the “onion-skinning” may resemble mantle cell lymphoma or marginal zone lymphoma. If the interfollicular stroma is abundant with occasional dysplastic DCs, the lesion may resemble CHL or angioimmunoblastic T-cell lymphoma (AITL) (Figure 19). If flow cytometry is available, the lack of a monoclonal B-cell population or an aberrant T-cell population with the morphologic features as described above should prompt the pathologist to consider the possibility of hv-CD or CHL. In this latter instance or when flow cytometry is not available, IHC will support or exclude the diagnosis of hv-CD. In the latter, the dysplastic stromal cells are vimentin+, SMA+/−, and CD21+/−, while CD15−, CD30−, and PAX5−. This immunophenotype rules out CHL. In addition, in hv-CD there remains—to some extent—a normal compartmentalization of T-cells predominantly located in interfollicular areas and of B-cells within follicles. There is no co-expression of CD5 or CD10 in B-cells and the mantle zone cells are negative for cyclin D1. Bcl-2 is negative in reactive germinal centers, however, careful evaluation of this marker is recommended since atretic follicles with depleted germinal centers may contain numerous bcl-2+ T-cells and bcl-2+ mantle zone cells that may appear as neoplastic bcl-2+ follicles of follicular lymphoma (44). A T-cell marker, CD10, bcl-6 and IgD are helpful in this situation to highlight the amount of germinal center T-cells, of mantle zone cells (IgD+) and the amount of preserved germinal center B-cells (CD10+/bcl-6+). CD21 and CD23 highlight the FDC with a concentric pattern at the mantle zones which is not typical of lymphomas. The diagnosis of AITL is ruled out by the lack of CD10, CD4, PD-1, bcl-6, CXCL13 and i-COS (T-follicular helper cell markers) on T-cells, and by the lack of expanded FDCM around blood vessels and coalescing with FDCs from lymphoid follicles (Figure 20), as well as by the absence of EBER+ cells.
The main features discussed in this section are summarized in Table 6.
Table 6
| Features | Hyaline-vascular CD | CHL | AITL |
|---|---|---|---|
| Clinical | • No age or gender predilection | • If NS-CHL, young F > M | • Usually, older adults |
| • No SVC syndrome unless mass >10 cm (bulky disease) | • Uncommon in anterior mediastinum | ||
| • May be accompanied by cervical or axillary LAPD | |||
| Imaging | • Mass of variable size with sharply demarcated borders | • Usually, well-circumscribed mass | • Anterior mediastinal mass |
| • May be asymptomatic or produce symptoms related to compression | • May show extension from adjacent structures | ||
| • Check clinical history for prior diagnosis of AITL | |||
| Morphology | • Onion-skinning | • Polymorphic infiltrate in nodules separated by fibrotic bands with scattered HRS cells | • Variable interfollicular expansion with increased vascularity and atypical lymphocytes, usually with clear cytoplasm |
| • Lollipop lesions | • Granulomas | • Variable number of eosinophils and plasma cells | |
| • Dysplastic FDCs in LFs and interfollicular areas | • No features of hyaline-vascular CD | • LFs may be decreased or pushed to the periphery | |
| • Variable interfollicular expansion with increased vascularity | • Variable number of eosinophils and plasma cells | • May contain HRS-like cells | |
| • Granulomas−/+ | |||
| • No HRS or HRS-like cells | |||
| • No eosinophils | |||
| • No increase in plasma cells | |||
| IHC | • T-cells > B-cells | • HRS cells: CD20− | • T-cells > B-cells |
| • T-cells: interfollicular | • PAX5+ dim | • Lymphoma cells: CD3+, other T-cell markers+ (may be loss) | |
| • B-cells: LFs | • CD30+ | • CD4+, CD8− | |
| • LFs: CD10+, bcl-6+ | • CD15+/− | • >2–3 TFH markers+ in T-cells (CD10, bcl-6, PD1, CD57, iCOS, CXCL13) | |
| • bcl-2−, Ki-67 high | • CD45− | • B-cells: EBER+ | |
| • CD21, CD23 concentric and prominent FDCM | • EBER−/+ | • CD21, CD23 expanded and prominent FDCM extending also to interfollicular areas and to blood vessels | |
| • Plasma cells: polytypic | • HRS-like cells may show T-cell phenotype or identical phenotype to CHL | ||
| • Dysplastic FDCs: CD21+, CD23+, CD20−, CD3−, CD30−, CD15−, PAX5− | |||
| • EBER− | |||
| Other | • Atrophic LFs with dysplastic FDCs may resemble follicular lymphoma, may also appear bcl2+ (see text) | • Interfollicular CHL (rare) can resemble hyaline-vascular CD | • If present, likely to be a known history of advanced stage disease |
| • Cases with stromal expansion may resemble CHL or AITL | • Consult hematopathology | • Consult hematopathology |
CD, Castleman disease; CHL, classic Hodgkin lymphoma; AITL, angioimmunoblastic T-cell lymphoma; NS-CHL, nodular sclerosis classic Hodgkin lymphoma; M, male; F, female; SVC, superior vena cava; LAPD, lymphadenopathy; FDCs, follicular dendritic cells; LFs, lymphoid follicles; HRS cells, Hodgkin/Reed-Sternberg cells; IHC, immunohistochemistry; FDCM, FDC meshworks; TFH, T-follicular helper cell.
Conclusions
The diagnosis of mediastinal lymphomas can be challenging in small biopsies, that are the current most common method of sampling of an anterior mediastinal mass. Because the initial clinical and/or imaging impression may not be that of lymphoma, these specimens tend to be first evaluated by cytopathologists, surgical pathologists, and thoracic pathologists rather than hematopathologists. For this reason, it is crucial for pathologists to have a practical diagnostic approach to these neoplasms, and to know their most common diagnostic pitfalls and main differential diagnoses. Once a proper initial work-up has been performed, a case can be transferred to a hematopathologist for further refinement of the diagnosis. We in no way imply that surgical and/or thoracic pathologists should start diagnosing lymphomas without proper expert consultation. Close communication with hematopathology colleagues is encouraged for continuous learning and improvement of clinical practices. In the same rank of importance, the appropriate triage of these samples, i.e., awareness to collect fresh material for flow cytometry, cytogenetic and/or molecular studies has a meaningful impact in the treatment of patients, as explained here for T-LBL confined to the mediastinum. By the same token, despite best efforts to establish a diagnosis, not uncommonly small biopsies may offer limited information to further classify an anterior mediastinal lesion. In this instance, close communication with clinical and surgical colleagues (multidisciplinary approach) is mandatory to determine next interventional and/or therapeutic decisions in the best interest of a patient.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://med.amegroups.com/article/view/10.21037/med-22-54/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://med.amegroups.com/article/view/10.21037/med-22-54/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Temes R, Chavez T, Mapel D, et al. Primary mediastinal malignancies: findings in 219 patients. West J Med 1999;170:161-6. [PubMed]
- Tateishi U, Müller NL, Johkoh T, et al. Primary mediastinal lymphoma: characteristic features of the various histological subtypes on CT. J Comput Assist Tomogr 2004;28:782-9. [Crossref] [PubMed]
- Temes R, Allen N, Chavez T, et al. Primary mediastinal malignancies in children: report of 22 patients and comparison to 197 adults. Oncologist 2000;5:179-84. [Crossref] [PubMed]
- Glick RD, La Quaglia MP. Lymphomas of the anterior mediastinum. Semin Pediatr Surg 1999;8:69-77. [Crossref] [PubMed]
- Bassan R, Maino E, Cortelazzo S. Lymphoblastic lymphoma: an updated review on biology, diagnosis, and treatment. Eur J Haematol 2016;96:447-60. [Crossref] [PubMed]
- Boddu P, Thakral B, Alhuraiji A, et al. Distinguishing thymoma from T-lymphoblastic leukaemia/lymphoma: a case-based evaluation. J Clin Pathol 2019;72:251-7. [Crossref] [PubMed]
- Patel JL, Smith LM, Anderson J, et al. The immunophenotype of T-lymphoblastic lymphoma in children and adolescents: a Children's Oncology Group report. Br J Haematol 2012;159:454-61. [Crossref] [PubMed]
- Pina-Oviedo S. Mediastinal Lymphoproliferative Disorders. Adv Anat Pathol 2021;28:307-34. [Crossref] [PubMed]
- Claudia G, Alexandra G. Challenging Diagnosis in NUT Carcinoma. Int J Surg Pathol 2021;29:722-5. [Crossref] [PubMed]
- Cazals-Hatem D, Lepage E, Brice P, et al. Primary mediastinal large B-cell lymphoma. A clinicopathologic study of 141 cases compared with 916 nonmediastinal large B-cell lymphomas, a GELA ("Groupe d'Etude des Lymphomes de l'Adulte") study. Am J Surg Pathol 1996;20:877-88. [Crossref] [PubMed]
- Grant C, Dunleavy K, Eberle FC, et al. Primary mediastinal large B-cell lymphoma, classic Hodgkin lymphoma presenting in the mediastinum, and mediastinal gray zone lymphoma: what is the oncologist to do? Curr Hematol Malig Rep 2011;6:157-63. [Crossref] [PubMed]
- O'Malley DP, Dogan A, Fedoriw Y, et al. American Registry of Pathology Expert Opinions: Immunohistochemical evaluation of classic Hodgkin lymphoma. Ann Diagn Pathol 2019;39:105-10. [Crossref] [PubMed]
- Bledsoe JR, Redd RA, Hasserjian RP, et al. The immunophenotypic spectrum of primary mediastinal large B-cell lymphoma reveals prognostic biomarkers associated with outcome. Am J Hematol 2016;91:E436-41. [Crossref] [PubMed]
- Calaminici M, Piper K, Lee AM, et al. CD23 expression in mediastinal large B-cell lymphomas. Histopathology 2004;45:619-24. [Crossref] [PubMed]
- Traverse-Glehen A, Pittaluga S, Gaulard P, et al. Mediastinal gray zone lymphoma: the missing link between classic Hodgkin's lymphoma and mediastinal large B-cell lymphoma. Am J Surg Pathol 2005;29:1411-21. [Crossref] [PubMed]
- O'Malley DP, Fedoriw Y, Weiss LM. Distinguishing Classical Hodgkin Lymphoma, Gray Zone Lymphoma, and Large B-cell Lymphoma: A Proposed Scoring System. Appl Immunohistochem Mol Morphol 2016;24:535-40. [Crossref] [PubMed]
- Jaffe ES, Stein H, Swerdlow SH, et al. B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classic Hodgkin lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al. editors. WHO classification of tumors of hematopoietic and lymphoid tissues (Revised 4th edition). Lyon: IARC; 2017:342-4.
- Sarkozy C, Molina T, Ghesquières H, et al. Mediastinal gray zone lymphoma: clinico-pathological characteristics and outcomes of 99 patients from the Lymphoma Study Association. Haematologica 2017;102:150-9. [Crossref] [PubMed]
- Kim HJ, Kim HK, Park G, et al. Comparative pathologic analysis of mediastinal B-cell lymphomas: selective expression of p63 but no GATA3 optimally differentiates primary mediastinal large B-cell lymphoma from classic Hodgkin lymphoma. Diagn Pathol 2019;14:133. [Crossref] [PubMed]
- Piña-Oviedo S, Moran CA. Primary Mediastinal Classical Hodgkin Lymphoma. Adv Anat Pathol 2016;23:285-309. [Crossref] [PubMed]
- Fraternali-Orcioni G, Falini B, Quaini F, et al. Beta-HCG aberrant expression in primary mediastinal large B-cell lymphoma. Am J Surg Pathol 1999;23:717-21. [Crossref] [PubMed]
- Hurley LJ, Burke CR, Shetty SK, et al. Elevated alpha-fetoprotein secondary to Hodgkin's lymphoma. Urology 1996;48:936-8. [Crossref] [PubMed]
- Marx A, Jain D, Marchevsky A, et al. Type B3 thymoma. In: Thoracic tumors. Lyon: IARC Press; 2021:340-4.
- Suster S, Moran CA. Histologic classification of thymoma: the World Health Organization and beyond. Hematol Oncol Clin North Am 2008;22:381-92. [Crossref] [PubMed]
- Dong M, Zhang X, Yang Z, et al. Patients over 40 years old with precursor T-cell lymphoblastic lymphoma have different prognostic factors comparing to the youngers. Sci Rep 2018;8:1088. [Crossref] [PubMed]
- Weissferdt A, Moran CA. Immunohistochemistry in the diagnosis of thymic epithelial neoplasms. Appl Immunohistochem Mol Morphol 2014;22:479-87. [Crossref] [PubMed]
- Toriyama A, Mori T, Sekine S, et al. Utility of PAX8 mouse monoclonal antibody in the diagnosis of thyroid, thymic, pleural and lung tumours: a comparison with polyclonal PAX8 antibody. Histopathology 2014;65:465-72. [Crossref] [PubMed]
- Moretti L, Medeiros LJ, Kunkalla K, et al. N-terminal PAX8 polyclonal antibody shows cross-reactivity with N-terminal region of PAX5 and is responsible for reports of PAX8 positivity in malignant lymphomas. Mod Pathol 2012;25:231-6. [Crossref] [PubMed]
- Go H, Cho HJ, Paik JH, et al. Thymic extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue: a clinicopathological and genetic analysis of six cases. Leuk Lymphoma 2011;52:2276-83. [Crossref] [PubMed]
- Inagaki H, Chan JK, Ng JW, et al. Primary thymic extranodal marginal-zone B-cell lymphoma of mucosa-associated lymphoid tissue type exhibits distinctive clinicopathological and molecular features. Am J Pathol 2002;160:1435-43. [Crossref] [PubMed]
- Shimizu K, Ishii G, Nagai K, et al. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) in the thymus: report of four cases. Jpn J Clin Oncol 2005;35:412-6. [Crossref] [PubMed]
- Arai H, Tajiri M, Kaneko S, et al. Two surgical cases of thymic MALT lymphoma associated with multiple lung cysts: possible association with Sjögren's syndrome. Gen Thorac Cardiovasc Surg 2017;65:229-34. [Crossref] [PubMed]
- Giroux Leprieur E, Antoine M, Gounant V, et al. Association between thymic MALT lymphoma and Sjögren's syndrome. Rev Pneumol Clin 2009;65:108-12. [Crossref] [PubMed]
- Sakamoto T, Yamashita K, Mizumoto C, et al. MALT lymphoma of the thymus with Sjögren's syndrome: biphasic changes in serological abnormalities over a 4-year period following thymectomy. Int J Hematol 2009;89:709-13. [Crossref] [PubMed]
- Takagi N, Nakamura S, Yamamoto K, et al. Malignant lymphoma of mucosa-associated lymphoid tissue arising in the thymus of a patient with Sjögren's syndrome. A morphologic, phenotypic, and genotypic study. Cancer 1992;69:1347-55. [Crossref] [PubMed]
- Weissferdt A, Moran CA. Primary MALT-type lymphoma of the thymus: a clinicopathological and immunohistochemical study of six cases. Lung 2011;189:461-6. [Crossref] [PubMed]
- Weissferdt A, Moran CA. Thymic hyperplasia with lymphoepithelial sialadenitis (LESA)-like features: a clinicopathologic and immunohistochemical study of 4 cases. Am J Clin Pathol 2012;138:816-22. [Crossref] [PubMed]
- Porubsky S, Popovic ZV, Badve S, et al. Thymic Hyperplasia with Lymphoepithelial Sialadenitis (LESA)-Like Features: Strong Association with Lymphomas and Non-Myasthenic Autoimmune Diseases. Cancers (Basel) 2021;13:315. [Crossref] [PubMed]
- Kuroki S, Nasu K, Murakami K, et al. Thymic MALT lymphoma: MR imaging findings and their correlation with histopathological findings on four cases. Clin Imaging 2004;28:274-7. [Crossref] [PubMed]
- Shimizu K, Yoshida J, Kakegawa S, et al. Primary thymic mucosa-associated lymphoid tissue lymphoma: diagnostic tips. J Thorac Oncol 2010;5:117-21. [Crossref] [PubMed]
- van Rhee F, Oksenhendler E, Srkalovic G, et al. International evidence-based consensus diagnostic and treatment guidelines for unicentric Castleman disease. Blood Adv 2020;4:6039-50. [Crossref] [PubMed]
- Wong RSM. Unicentric Castleman Disease. Hematol Oncol Clin North Am 2018;32:65-73. [Crossref] [PubMed]
- Wang W, Medeiros LJ. Castleman Disease. Surg Pathol Clin 2019;12:849-63. [Crossref] [PubMed]
- Pina-Oviedo S, Miranda RN, Lin P, et al. Follicular lymphoma with hyaline-vascular Castleman-like features: analysis of 6 cases and review of the literature. Hum Pathol 2017;68:136-46. [Crossref] [PubMed]
Cite this article as: Pina-Oviedo S, Pavlisko E, Glass C, DiBernardo L, Sporn T, Roggli V. Diagnostic approach to prevascular (anterior) mediastinal lymphomas: when thoracic pathology meets hematopathology. Mediastinum 2023;7:35.


