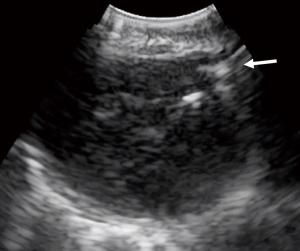
Narrative review of tools for endoscopic ultrasound-guided biopsy of mediastinal nodes
Introduction
Lung cancer remains the leading cause of death among all malignancies worldwide, representing 18.4% of all cancer-related mortality (1). Although transbronchial needle aspiration (TBNA) has been part of the diagnostic algorithm for lung cancers for decades (2,3), real-time ultrasound-guided TBNA has become mainstream only recently. Endobronchial ultrasound (EBUS) was first introduced in 1992 as a 360° rotating radial probe (4) which allowed visualization of mediastinal structures, vasculature, lymph nodes and tumors adjacent to the central airways. This technology however, was mostly a novelty due to the inability to perform concurrent biopsy of the visualized structures. The linear EBUS bronchoscope, with a separate working channel that allowed for real-time EBUS-TBNA, was introduced in 2002 (5) and rapidly became an essential tool for both diagnosing and staging of lung cancer. Given its utility in assessing mediastinal structures, EBUS has also gained a prominent role in the evaluation of non-cancer etiologies of mediastinal and hilar lymphadenopathy (6).
EBUS-TBNA has become the preferred method of mediastinal staging of lung cancer as per the American College of Chest Physicians (7). Since the introduction of EBUS, many advances in techniques and tools for EBUS-guided biopsy have emerged over the past several years. Here, we review the recent advancements in equipment and tools related to linear EBUS-guided bronchoscopy and biopsy. We present the following article in accordance with the NARRATIVE REVIEW reporting checklist (available at http://dx.doi.org/10.21037/med-20-25).
Data review
Literature search was done via the PubMed® database. We included all types of articles and study design, including original research, meta-analyses, reviews, and abstracts. Only studies published in English were considered.
Equipment
Bronchoscopes
Although endoscopic ultrasound (EUS) was developed in the 1980s, it was not until years later that EBUS bronchoscopes became readily available due to the technical challenges in adapting this technology for the airways (8,9). After multiple prototypes, the linear (convex-probe, cp) EBUS bronchoscope was introduced into clinical practice in 2002 (5). The linear EBUS bronchoscopes have an elongated curved array ultrasound transducer at the distal tip with a camera and a light source that is offset at an angle (10-12). It allows for both direct visualization within the airways as well as ultrasonographic view of the lymph node, needle device, and vasculature with color flow doppler. Currently on the market, there are three companies that produce EBUS bronchoscopes: Olympus, Fuji and Pentax (Table 1).

Full table
There are limited studies evaluating the different bronchoscopes and no head-to-head trials comparing the three brands of bronchoscopes to date. A major difference between the Olympus EBUS scope and those produced by Pentax and Fuji is the imaging system. Olympus uses a fiberoptic system for transmitting the bronchoscopic images while the other manufacturers utilize a charge coupled device (CCD) chip within the distal tip of the scope. The images produced from CCD are higher quality digital images (13). However, the CCD chip has a larger footprint than the fiberoptic system, necessitating a smaller working channel at 2.0 mm, except for the most recent Pentax EBUS bronchoscope (EB19-J10U), which has a 2.2 mm working channel similar to the Olympus bronchoscope. The larger working channel allows for use of 19G and 21G needles which are incompatible with the other systems and may allow for enhanced suction capability. Importantly, it is worth mentioning that in general, due to the anterior location and smaller size of the working channel, the EBUS bronchoscopes have limited suction capacity. Thus, it is recommended to use a dedicated therapeutic bronchoscope for the clearance of secretions before airway inspection and after performing EBUS-TBNA.
The other differences include the smaller outer diameter of the Fuji scope which could make it more comfortable for patients, as well as the 10 degree forward oblique view as opposed to the 35–45 degree forward oblique view of Olympus and Pentax (14). This allows simultaneous visualization of the distal end of the scope as well as the ultrasound image, providing a familiar view for seasoned bronchoscopists interested in adding EBUS to their skill-set.
Ultrasound processors
Each EBUS bronchoscope requires a different ultrasound processor. From Olympus, there are the EU-ME1 and EU-ME2 processors. The EU-ME1 was launched in 2009 and at the time, was the first processor to combine electronic and mechanical scanning into one device, so that multiple scopes and ultrasound probes for both pulmonary and GI procedures, could all be connected to the same unit. Subsequently, it was replaced by the EU-ME2 ultrasound processor, which incorporated new features. With EU-ME2, it improved on imaging display modes and integrated the ability to perform contrast enhanced harmonic imaging and elastography. The Olympus ultrasound processors have cross-departmental functionality, and within pulmonology, can be used with both linear and radial EBUS probes, which can lead to cost-savings and procedural efficiencies.
Fuji’s ultrasound processor, SU-1, also has multiple imaging modes, including elastography, color doppler and harmonic imaging. Similar to Olympus, the Fuji processor can also be used for both GI and pulmonary procedures; however, for EBUS, it can only be used with the linear probe and is not compatible with radial probe EBUS. One of the unique features of Fuji’s imaging technology is the sound speed correction, in which images are reconstructed using an estimated optimal sound speed within the body, to provide improved resolution.
Similar to the EBUS bronchoscopes, there are limited studies comparing the ultrasound processor units. The choice of ultrasound processor is directly related to the choice of EBUS bronchoscope.
Elastography
One of the features of recent ultrasound processors is the ability to display elastography. Ultrasound elastography is a technique that measures the stiffness and compressibility of tissue. Malignant tissue is often stiffer than normal tissues, so elastography has been used in assessing malignant thyroid (15) and breast lesions (16,17) in an attempt to potentially avoid biopsies. More recently, elastography has been used with EBUS to evaluate lymph nodes (18,19) and has been shown to be able to identify malignant lymph nodes (20,21). However, calcifications or fibrosis can also lead to stiffer tissues. Elastography has been well documented in use for liver fibrosis (22,23), so fibrotic or densely calcified lymph nodes may be falsely characterized as neoplastic. Furthermore, necrotic or highly vascular lymph nodes may be falsely deemed benign by elastography (24). Although elastography can provide additional supportive information in terms of which lymph nodes to target, ultimately biopsy to obtain tissue is still warranted for diagnosis and staging.
Needles
Overview
Since the introduction of EBUS-TBNA, multiple needles have emerged on the market. Needles of various sizes and designs are available for tissue acquisition (Figure 1). Currently, there are three companies that produce the majority of EBUS-TBNA needles: Olympus, Boston Scientific and Cook.
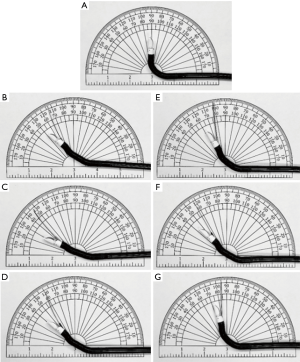
Olympus has designed the ViziShot and ViziShot 2 needles. The ViziShot includes a 21-gauge (21G) and 22G, while the ViziShot 2 class includes a 21G and 22G as well as a 19G Flex needle (Olympus Respiratory America, Redmond, WA). Due to the stiffness of the needle, there is often limited flexion of the distal tip of the EBUS bronchoscope when the needle is in the working channel (Table 2) (25). The second generation ViziShot 21G and 22G needles incorporate spiral laser cuts on the needle surface, which significantly increase flexibility and ultra-sonographic needle visualization compared to the original ViziShot design. The ViziShot2 Flex 19G needle has the greatest flexibility, which increases the ability to sample from locations which require more scope angulation, such as 4L (25) or within the upper lobes (26). Additionally, with its larger lumen, the 19G needle was marketed as one which can obtain larger amounts of tissue to allow for enhanced histological analysis.

Full table
Boston Scientific produces the Expect 22G and 25G TBNA needles, Acquire fine needle biopsy (FNB) needle in 22G and 25G, and the CoreDx mini-forceps (Boston Scientific, Watertown, MA). The Expect needle is made from cobalt chromium, as opposed to stainless steel, which enhances its ability to penetrate through stiffer tissue or cartilage and withstand multiple passes. This may shorten procedural time (27) as less time is spent on failed passes or exchanging needles. The Acquire needle has a Franseen needle tip, which consists of 3-prongs that increases the overall cutting surface and is designed for enhanced tissue acquisition while minimizing fragmentation of the specimen. Additionally, the 3 needle points provide greater control at the puncture site. CoreDx mini-forceps is used in conjunction with EBUS-TBNA to obtain additional biopsy samples for histology evaluation.
Cook Medical has the EchoTip Ultra TBNA needles and the EchoTip ProCore FNB needles, both which include a 22G and 25G (Cook Medical, Bloomington, IN). The unique feature of the ProCore needle is a Menghini bevel with a lateral cutout just proximal to the tip of the needle, called a core trap, which is designed to facilitate additional tissue sampling. With the core trap, despite the smaller size of the 25G ProCore needle, it is able to obtain sufficient samples when compared to the 22G (28).
21G and 22G needles
Historically, the EBUS-TBNA 22G needle was developed first, followed shortly by the 21G needle. Multiple studies comparing the diagnostic yield of both sizes of needles, have not found a significant difference (29-31). There is a high success rate of diagnosis with both, with a diagnostic yield for malignancy of 96.6% with 21G needles and 95.3% with 22G needles (29) (Figures 2,3). In a large retrospective review of over 1,200 patients who underwent EBUS-TBNA, there was similarly no difference in diagnostic yield or specimen adequacy between the 21G and 22G needles (32).
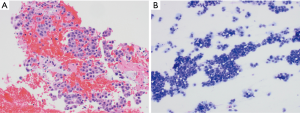
Although both needles achieved similar diagnostic yield, there was better characterization of non-malignant diseases and histologic preservation of malignant diseases with the 21G needle (29,30). Despite better characterization of benign diseases, especially sarcoidosis, with the 21G needle (29) the overall diagnostic ability of both the 21 and 22G needles for sarcoidosis is not as robust as in non-small cell lung cancer (NSCLC). In two studies by Herth and colleagues, they found the diagnostic ability of the 22G needle for sarcoidosis to be 24–33% (33,34), while a different study found a higher rate of sarcoid diagnosis at 61%, but still much lower than the rate of NSCLC diagnosis at 80% (35). A systematic review of 15 studies evaluating use of EBUS-TBNA in sarcoidosis found a pooled diagnostic accuracy of 79%, with the yield ranging between 54–93% (36), highlighting the substantial variability in diagnostic sensitivities reported for sarcoidosis. The use of EBUS-TBNA to diagnose lymphoproliferative malignancies has also had similar pitfalls (34), with diagnostic sensitivities ranging from 38–90% (37-39). A meta-analysis of 14 studies evaluating EBUS-TBNA with 21G or 22G needles in diagnosing lymphoma reported a pooled sensitivity of 66% (40).
19G needle
Due to the limitations of the 21 and 22G needles, most notably the challenges with diagnostic accuracy in lymphoma and sarcoidosis, additional tools were created to address this issue. In 2015, the Olympus ViziShot 2 Flex 19G needle was introduced which was designed to provide larger tissue samples as well as greater flexibility, and several studies have evaluated the performance of the 19G needle in both malignant and benign disease.
Tyan et al. reported a diagnostic yield of 89% (42/47 patients) with the Vizishot Flex 19G needle (25). The majority of cases were done with conscious sedation and application of needle vacuum-suction during lymph node sampling. Despite the larger bore of the 19G needle, there were no complications seen except for mild bleeding, which did not require intervention other than local suctioning. Similarly, another group reported 13 patients who underwent EBUS-TBNA with 19G needle, in whom minor bleeding was seen in one patient which self-resolved (41).
Multiple studies evaluating the performance of 19G needle have shown no significant difference in diagnostic yield between the 19G needle, as compared to the 21G or 22G needles (42-44). Specimens from the 19G needle were noted to be bloodier overall (59% with 19G vs. 19% with 22G) (43). However, this did not affect the diagnostic yield (45). Of interest, one study showed the 19G needle achieved less adequate samples compared to 22G (46% with 19G vs. 73% with 22G) (43), while another study showed the 19G needle achieved large volumetric and cohesive tissue samples (44).
Perhaps most significantly, a randomized controlled trial by Dooms et al. found no superiority with 19G in procuring tissue core for cell block (45). However, the 19G needle did achieve larger tissue surface area which may be beneficial for molecular marker testing in NSCLC. Subsequent prospective randomized trials comparing EBUS-TBNA samples obtained from 19G and 22G concluded that specimens acquired from the 19G needle had significantly more tissue, as well as significantly more tumor cells (46). These benefits were seen without any increase in complications (46,47). With advanced molecular testing in NSCLC becoming more customary, the 19G needle may obtain more tissue to support these tests (48).
Although overall diagnostic yield in NSCLC appeared to be similar with 19G as compared to 22G, there are a subset of conditions which require tissue architecture to diagnose and may benefit from larger bore needles. Several studies have compared the diagnostic yield in sarcoidosis and lymphoma when using different needle sizes. Pooled results from these studies show an overall increased diagnostic yield in both sarcoidosis and lymphoma with the 19G needle or mini-forceps, which are classified as “histology” tools, as compared to the 21G and 22G needles, which are classified as “cytology” tools (24). With “histology” tools, the diagnostic yield was 68% vs. 51% with “cytology” tools for sarcoidosis. Similarly, in lymphoma, the diagnostic yield with “histology” tools was 63% vs. 21% with “cytology” tools (24).
25G needle
There are limited data on the use of 25G needles in EBUS-TBNA. A retrospective study comparing the efficacy of the Boston Scientific Expect 25G to the Olympus ViziShot 22G needles in EBUS-TBNA, demonstrated comparable diagnostic accuracy and specimen adequacy (92%, 73/79 with both needles) (49). In the setting of next-generation sequencing, Stoy et al. showed that the 25G needle was able to obtain adequate tissue and similar diagnostic yield as the 22G needle (50). Of note, given the small caliber of the 25G needle, it is vulnerable to clotting after repeated lymph node sampling which could affect yield, but this may be countered with wiping the stylet with heparin after each use. Heparin priming of the needle did not increase blood contamination of the specimen, or negatively affect cytological or histological analysis (51).
Most of the experience with 25G needles is in the gastrointestinal (GI) literature for endoscopic ultrasound-fine needle aspiration (EUS-FNA) of pancreatic lesions. A recent meta-analysis of over 500 studies comparing 22G vs. 25G needles in EUS-FNA found no significant difference in diagnostic accuracy between the two needles (52). However, a meta-analysis from 2013 found that while the specificity was similar between both 22G and 25G needles (1.00 in 22G vs. 0.97 in 25G, P=0.97), the sensitivity for diagnosing malignancy was higher with the 25G needle (0.85 in 22G vs. 0.93 in 25G, P=0.0003) (53). The increased sensitivity with the 25G needle might theoretically be due to fewer bloody aspirates, facilitating cytological interpretation without compromising cellular yield, as well as both greater needle flexibility and better to-and-fro traversal passage of the needle to the target lesion. Importantly, there was a discrepancy between diagnostic yield obtained from rapid on-site evaluation (ROSE) and cell blocks when the 25G needle was used for EUS-FNA. Although ROSE established malignancy in 100% of patients, it only resulted in a diagnostic cell block in 81% of cases with a 25G needle (54). Although these studies are from GI procedures, these results may extrapolate to EBUS-TBNA and may be important when choosing needle size.
Fine needle aspiration (FNA) versus Fine needle biopsy (FNB)
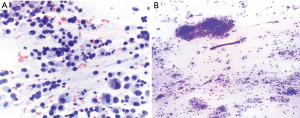
FNB needles were designed to obtain larger tissue and core samples for improved histological and cytological evaluation, compared to fine needle aspiration needles. The choice between FNA and FNB needles has been evaluated, mostly in the GI literature as well. A recent study assessing the efficacy of the Acquire FNB needle in EUS-FNB evaluation of intra-abdominal lesions found that a core was present in 90% of the samples (55). In a small prospective study of patients with pancreatic lesions, individuals were randomized to either EUS-FNA with Olympus 22G needle or EUS-FNB with Cook EchoTip ProCore 22G needle. Diagnostic yield was the same in both groups at around 83%, but definitive diagnosis was established with fewer punctures with FNB as compared to FNA (1.11 vs. 1.83, P<0.05) (56), which has been consistent in other studies as well (57). Similarly, a meta-analysis of nine studies comparing the ProCore FNB needle to standard FNA needles found no difference in diagnostic adequacy or accuracy, but the number of passes required for diagnosis was lower with FNB (58) (Figure 4).
Intranodal forceps, or mini-forceps, biopsy (IFB) were introduced as a means to obtain larger tissue samples. With EBUS-IFB, mini-forceps are passed through the bronchoscope into the target lymph node following a standard TBNA puncture (Figure 5, Video 1). Mini-forceps has a role in obtaining larger amounts of tissue which may aid in preserving histopathology for diagnosis of sarcoidosis and lymphoma (Figure 4B). With the use of IFB as compared to TBNA, the diagnostic yield for both sarcoidosis and lymphoma were increased (34,59). One study showed the rate of diagnosis for sarcoidosis increased from 24% to 88% and for lymphoma from 11% to 81% between 22G TBNA and IFB respectively (34). In malignant disease, IFB also has a role in obtaining additional tissue for molecular marker analysis (60). Despite larger samples, the safety profile of EBUS-IFB is similar to EBUS-TBNA, with an overall complication rate of 1.5% and no deaths in the published cases of EBUS-IFB (60).
Tool selections
With a multitude of TBNA needles to choose from, there is no clear “best needle” and each size and type of needle may have a role depending on the clinical situation. There was a recent survey of bronchoscopists evaluating different EBUS-TBNA needles which showed that providers rated the Boston Scientific Expect 25G and Olympus ViziShot 22G highest for ease of insertion, and Boston Scientific Expect 22G for durability, but the highest overall rated needle was the Olympus ViziShot 22G (61). The variability and lack of overall consensus regarding needles highlights the subjective nature of needle preference, and the unique design features of each type of needle may be best suited for different clinical scenarios.
Often times the ultimate selection in tools may depend on a variety of factors, including local availability, operational costs, sampling location and histopathologic diagnostic requirements. Given the wide selection of needles, a single medical center may not stock all the options. Therefore, operators will need to be aware and comfortable with the use of the needles at their respective institutions.
Cost analysis
EBUS significantly reduced the cost of both diagnosing and staging lung cancer when compared to surgical staging. The ASTER study randomized patients with potentially resectable NSCLC to surgical staging or EBUS-TBNA, with subsequent surgical staging if EBUS-TBNA was negative (62). With this strategy, the average cost in the surgical arm was 10,459£ compared to 9,713£ with EBUS. The majority of the cost savings was from preventing unnecessary surgery, as mediastinoscopies were avoided in close to half the patients and thoracotomies were reduced by 11%. In addition, the sensitivity for nodal metastases (N2 or N3) was higher with EBUS at 94% compared to mediastinoscopy at 79% (62).
When EBUS-TBNA was compared to blind TBNA, there was still a cost savings associated with EBUS when accounting for the subsequent necessary surgical procedures for complete staging (63). With EBUS, more lymph nodes were sampled and overall diagnostic yield with EBUS was higher at 87%, compared to only 59% in the blind TBNA group. Therefore, there were less patients in the EBUS group who ultimately required surgery compared to those who received blind TBNA (11% vs. 24%), and the overall estimated average cost savings with EBUS was about $787 per procedure. Another study estimated an average savings of 1,450€ with EBUS-TBNA (64).
In addition to hospital financial savings, there was also time savings for the patients. The median time to treatment decision was 14 days with EBUS-TBNA as compared to 29 days with other methods, which included CT-guided biopsy, mediastinoscopy and positron emission tomography (PET) scan (65). PET scan previously had a prominent role in lung cancer staging, but studies have shown a high false negative rate with PET/CT staging. In 113 patients with NSCLC and radiographic N0 disease who underwent EBUS, there were 20 patients who were upstaged to N2 disease (66). Similarly, in 220 patients with N0 disease, there were a total of 45 patients (20.5%) who were upstaged by EBUS or surgery (67). Given the high false negative rate with PET/CT imaging, as well as the importance of obtaining tissue, EBUS-TBNA still has a role in radiographic N0 disease.
EBUS should be the initial diagnostic procedure of choice in patients with accessible mediastinal masses or lymphadenopathy. There was an average delay in diagnosis of 18 days in patients with small cell lung cancer who underwent other diagnostic procedures prior to EBUS (68). EBUS is an outpatient procedure with quick recovery time, so patients who received staging with EBUS as compared to surgery rated their quality of life higher as well (62). The use of EBUS-TBNA in lung cancer staging and diagnosis is cost-effective, limits treatment delays for patients and has superior diagnostic performance compared to PET/CT scan which can dramatically alter treatment decisions and prognosis (69).
Future directions
Since the introduction of EBUS about 20 years ago, its utility has grown rapidly from mediastinal lymph node sampling to now include a large variety of other clinical situations.
Peripheral lung nodules, which previously were deemed not amenable to EBUS are becoming increasingly accessible via new technology. Electromagnetic navigational bronchoscopy with cone beam CT scan in conjunction with EBUS has been successfully used to biopsy peripheral or difficult to access pulmonary nodules (70). Additionally, the iNod system (Boston Scientific, Watertown, MA) allows for real-time biopsy of peripheral nodules under radial EBUS visualization. In addition to lymph node and lung nodule biopsies, there have also been reports of EBUS being used in thyroid biopsies for lesions not amenable to percutaneous biopsy (71,72). Beyond diagnostics, EBUS may also have a therapeutic role in facilitating lung cancer treatment via placement of fiducial markers to guide radiotherapy (73,74), transbronchial needle injection to deliver chemotherapy locally (75,76) and there is ongoing research regarding linear EBUS-guided ablation of central lesions.
There may also be a role for EBUS in non-biopsy and non-malignant clinical scenarios (77). Given frequent barriers in diagnosing pulmonary emboli (PE) and the ability of EBUS to view major mediastinal blood vessels, a pilot study was done in 2009 to evaluate the feasibility of EBUS in diagnosing central PE (78). In 32 patients, EBUS detected PE in 96% of the cases that were confirmed on CT scan. There is currently an ongoing clinical trial (NCT04047784) evaluating the role of EBUS in diagnosing PE in critically ill patients. EBUS may also develop a role in examining other mediastinal structures. There are cases of EBUS being used to facilitate drainage of mediastinal and bronchogenic cysts (79,80), as well as reports describing pericardial effusions that are treated by EBUS-guided pericardiocentesis (81,82).
Conclusions
The field of interventional pulmonology is rapidly growing. EBUS, which is one of the landmark advancements in the field, has become widespread and indispensable in a short timeframe and continues to find a role in new clinical scenarios. With the increasing use of EBUS, major advancements in the equipment and tools for EBUS have been developed over the last several years. While randomized controlled clinical trials to evaluate the efficacy and outcomes of these novel EBUS devices are still needed, the rapid rate of growth and innovation hopefully signifies the trajectory of advancements still to come in interventional pulmonology
Acknowledgments
Special thanks to Dr. Vera Vavinskaya and Dr. Jeenal Gordhandas from the Department of Pathology at University of California San Diego for providing the histopathology slides from EBUS specimens.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Angelo Carretta) for the series “Minimally Invasive (Endoscopic) Mediastinal Staging of Lung Cancer” published in Mediastinum. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Reporting Checklist: The authors have completed the NARRATIVE REVIEW reporting checklist. Available at http://dx.doi.org/10.21037/med-20-25
Peer Review File: Available at http://dx.doi.org/10.21037/med-20-25
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-25). The series “Minimally Invasive (Endoscopic) Mediastinal Staging of Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. JDC reports to receive honoraria from Cook Medical, outside the submitted work; GC reports that he has served as a consultant to Boston Scientific; Medtronic plc; Pinnacle Biologics, Inc.; and Restor3D and has received research funding from Intuitive Surgical Inc and Pinnacle Biologics, Inc., outside the submitted work. The authors have no other conflicts of interest to declare.
Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Yang H, Zhang Y, Wang K, et al. Transbronchial needle aspiration: development history, current status and future perspective. J Thorac Dis 2015;7:S279-86. [PubMed]
- Wang KP. Turner, Symanowski J. A retrospective review of different methods of endobronchial ultrasound-guided transbronchial needle aspiration: a preliminary study. J Bronchology Interv Pulmonol 2011;18:94-6. [Crossref] [PubMed]
- Hürter T, Hanrath P. Endobronchial sonography: feasibility and preliminary results. Thorax 1992;47:565-7. [Crossref] [PubMed]
- Yasufuku K, Chiyo M, Sekine Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest 2004;126:122-8. [Crossref] [PubMed]
- Wahidi MM, Herth F, Yasufuku K, et al. Technical aspects of endobronchial ultrasound-guided transbronchial needle aspiration: CHEST guideline and expert panel report. Chest 2016;149:816-35. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer. Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013;143:e211S-e250S.
- Gomez M, Silvestri G. Endobronchial ultrasound for the diagnosis and staging of lung cancer. Proc Am Thorac Soc 2009;6:180-6. [Crossref] [PubMed]
- Becker HD. Short history of the development of endobronchial ultrasound – a story for success. From Clinical Chest Ultrasound: From the ICU to the Bronchoscopy Suite. Prog Respir Res. Basel, Karger, 2009, vol 37, Chapter 14, pp 128-139.
- Yasufuku K and Nakajima T. Convex probe endobronchial ultrasound. From Clinical Chest Ultrasound: From the ICU to the Bronchoscopy Suite. Basel, Karger: Prog Respir Res, 2009, vol 37, Chapter 16, pp 147-152.
- Davidsen R. Curved 2-D array ultrasound transducer and method for volumetric imaging. J Acoust Soc Am 2011;130:2315. [Crossref]
- Belanger AR, Akulian JA. An update on the role of advanced diagnostic bronchoscopy in the evaluation and staging of lung cancer. Ther Adv Respir Dis 2017;11:211-21. [Crossref] [PubMed]
- Kato H, Kobayashi T, Konaka C. Controversy: Video (CCD) flexible bronchoscope versus standard flexible bronchoscope: pro videobronchoscope. J Bronchology Interv Pulmonol 1995;2:328-30.
- Xiang Y, Zhang F, Akulian J, et al. EBUS-TBNA by a new Fuji EBUS scope (with video). J Thorac Dis 2013;5:36-9. [PubMed]
- Sun J, Cai J, Wang X. Real-time ultrasound elastography for differentiation of benign and malignant thyroid nodules: a meta-analysis. J Ultrasound Med 2014;33:495-502. [Crossref] [PubMed]
- Gong X, Xu Q, Xu Z, et al. Real-time elastography for the differentiation of benign and malignant breast lesions: a meta-analysis. Breast Cancer Res Treat 2011;130:11-8. [Crossref] [PubMed]
- Sadigh G, Carlos RC, Neal CH, et al. Accuracy of quantitative ultrasound elastography for differentiation of malignant and benign breast abnormalities: a meta-analysis. Breast Cancer Res Treat 2012;134:923-31. [Crossref] [PubMed]
- Nakajima T, Inage T, Sata Y, et al. Elastography for predicting and localizing nodal metastases during endobronchial ultrasound. Respiration 2015;90:499-506. [Crossref] [PubMed]
- Izumo T, Sasada S, Chavez C, et al. Endobronchial ultrasound elastography in the diagnosis of mediastinal and hilar lymph nodes. Jpn J Clin Oncol 2014;44:956-62. [Crossref] [PubMed]
- Ying L, Hou Y, Zheng HM, et al. Real-time elastography for the differentiation of benign and malignant superficial lymph nodes: a meta-analysis. Eur J Radiol 2012;81:2576-84. [Crossref] [PubMed]
- Verhoeven RL, de Korte CL, van der Heijden EH. Optimal endobronchial ultrasound strain elastography assessment strategy: an explorative study. Respiration 2019;97:337-47. [Crossref] [PubMed]
- Lee SM, Lee JM, Kang H, et al. Liver fibrosis staging with a new 2D-shear wave elastography using comb-push technique: applicability, reproducibility, and diagnostic performance. PLoS One 2017;12:e0177264 [Crossref] [PubMed]
- Barr RG, Ferraioli G, Palmeri ML, et al. Elastography assessment of liver fibrosis: society of radiologists in ultrasound consensus conference statement. Radiology 2015;276:845-61. [Crossref] [PubMed]
- He T, Mehta A. Linear endobronchial ultrasound: what’s new? Semin Respir Crit Care Med 2018;39:649-60. [Crossref] [PubMed]
- Tyan C, Patel P, Czarnecka K, et al. Flexible 19-gauge endobronchial ultrasound guided transbronchial needle aspiration needle: first experience. Respiration 2017;94:52-7. [Crossref] [PubMed]
- Jalil BA, Yasufuku K, Khan A. Uses, limitations, and complications of endobronchial ultrasound. Proc (Bayl Univ Med Cent) 2015;28:325-30. [Crossref] [PubMed]
- Uchimura K, Yamasaki K, Tachiwada T, et al. Utility and safety of cobalt chromium endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA). Respirology 2018;23:AO011,8.
- Matsumoto Y, Tanaka M, Nakai T, et al. The potential of a new 25-gauge needle with a core-trap as transbronchial needle biopsy: a pilot study. Chest 2019;155:260A. [Crossref]
- Jeyabalan A, Shelley-Fraser G, Medford AR. Impact of needle gauge on characterization of endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) histology samples. Respirology 2014;19:735-9. [Crossref] [PubMed]
- Nakajima T, Yasufuku K, Takahashi R, et al. Comparison of 21-gauge and 22-gauge aspiration needle during endobronchial ultrasound-guided transbronchial needle aspiration. Respirology 2011;16:90-4. [Crossref] [PubMed]
- Oki M, Saka H, Kitagawa C, et al. Randomized study of 21-gauge versus 22-gauge endobronchial ultrasound-guided transbronchial needle aspiration needles for sampling histology specimens. J Bronchology Interv Pulmonol 2011;18:306-10. [Crossref] [PubMed]
- Yarmus LB, Akulian J, Lechtizin N, et al. Comparison of 21-gauge and 22-gauge aspiration needle in endobronchial ultrasound-guided transbronchial needle aspiration: results of the American College of Chest Physicians Quality Improvement Registry, Education and Evaluation Registry. Chest 2013;143:1036-43. [Crossref] [PubMed]
- Herth FJ, Eberhardt R, Vilmann P, et al. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax 2006;61:795-8. [Crossref] [PubMed]
- Herth FJ, Morgan R, Eberhardt R, et al. Endobronchial ultrasound-guided miniforceps biopsy in the biopsy of subcarinal masses in patients with low likelihood of non-small cell lung cancer. Ann Thorac Surg 2008;85:1874-8. [Crossref] [PubMed]
- Darwiche K, Freitag L, Nair A, et al. Evaluation of a novel endobronchial ultrasound-guided lymph node forceps in enlarged mediastinal lymph nodes. Respiration 2013;86:229-36. [Crossref] [PubMed]
- Agarwal R, Srinivasan A, Aggarwal A, et al. Efficacy and safety of convex probe EBUS-TBNA in sarcoidosis: A systematic review and meta-analysis. Respir Med 2012;106:883-92. [Crossref] [PubMed]
- Iqbal S, DePew ZS, Kurtin PJ, et al. Endobronchial ultrasound and lymphoproliferative disorders: a retrospective study. Ann Thorac Surg 2012;94:1830-4. [Crossref] [PubMed]
- Kennedy MP, Jimenez CA, Bruzzi JF, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lymphoma. Thorax 2008;63:360-5. [Crossref] [PubMed]
- Steinfort DP, Conron M, Tsui A, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the evaluation of suspected lymphoma. J Thorac Oncol 2010;5:804-9. [Crossref] [PubMed]
- Labarca G, Sierra-Ruiz M, Kheir F, et al. Diagnostic accuracy of endobronchial ultrasound transbronchial needle aspiration in lymphoma. A systematic review and meta-analysis. Ann Am Thorac Soc 2019;16:1432-9. [Crossref] [PubMed]
- Trisolini R, Natali F, Ferrari M, et al. Endobronchial ultrasound-guided transbronchial needle aspiration with the flexible 19-gauge needle. Clin Respir J 2018;12:1725-31. [Crossref] [PubMed]
- Porfyridis I, Frangopoulos F, Vogazianos P, et al. Comparison of diagnostic performance of 19-gauge and 21-gauge needles during endobronchial ultrasound-guided transbronchial needle aspiration. Eur Resp J 2017;50:PA823.
- Chaddha U, Ronaghi R, Elatre W, et al. Comparison of sample adequacy and diagnostic yield of 19- and 22-G EBUS-TBNA needles. J Bronchology Interv Pulmonol 2018;25:264-8. [Crossref] [PubMed]
- Kinoshita T, Ujiie H, Schwock J, et al. Clinical evaluation of the utility of a flexible 19-gauge EBUS-TBNA needle. J Thorac Dis 2018;10:2388-96. [Crossref] [PubMed]
- Dooms C, Vander Borght S, Yserbyt J, et al. A randomized clinical trial of Flex 19G needles versus 22G needles for endobronchial ultrasonography in suspected lung cancer. Respiration 2018;96:275-82. [Crossref] [PubMed]
- Wolters C, Darwiche K, Franzen D, et al. A prospective, randomized trial for the comparison of 19-G and 22-G endobronchial ultrasound guided transbronchial aspiration needles;introducing a novel end point of sample weight corrected for blood content. Clin Lung Cancer 2019;20:e265-273. [Crossref] [PubMed]
- Tenda E, Aboelhassan A, Kontogianni K, et al. Endobronchial ultrasound transbronchial needle aspiration (EBUS-TBNA) versus flexible 19G endobronchial ultrasound transbronchial needle (Flex 19G EBUS-TBNA) in the assessment of mediastinal and hilar lymphadenopathy: a randomised trial. Eur Resp J 2019;54:PA324.
- Garrison G, Leclair T, Balla A, et al. Use of an additional 19-G EBUS-TBNA needle increases the diagnostic yield of EBUS-TBNA. J Bronchology Interv Pulmonol 2018;25:269-73. [Crossref] [PubMed]
- Di Felice C, Young B, Matta M. Comparison of specimen adequacy and diagnostic accuracy of a 25-gauge and 22-gauge needle in endobronchial ultrasound-guided transbronchial needle aspiration. J Thorac Dis 2019;11:3643-9. [Crossref] [PubMed]
- Stoy SP, Segal JP, Mueller J, et al. Feasibility of endobronchial ultrasound-guided transbronchial needle aspiration cytology specimens for next generation sequencing in non-small cell lung cancer. Clin Lung Cancer 2018;19:230-8.e2. [Crossref] [PubMed]
- Diehl DL, Mok SR, Khara HS, et al. Heparin priming of EUS-FNA needles does not adversely affect tissue cytology or immunohistochemical staining. Endosc Int Open 2018;6:E356-62. [Crossref] [PubMed]
- Guedes HG, Moura DTH, Duarte RB, et al. A comparison of the efficiency of 22G versus 25G needles in EUS-FNA for solid pancreatic mass assessment: A systematic review and meta-analysis. Clinics (Sao Paulo) 2018;73:e261 [Crossref] [PubMed]
- Madhoun MF, Wani SB, Rastogi A, et al. The diagnostic accuracy of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of solid pancreatic lesions: a meta-analysis. Endoscopy 2013;45:86-92. [Crossref] [PubMed]
- Varadarajulu S, Bang JY, Holt BA, et al. The 25-gauge EUS-FNA needle: Good for on-site but poor for off-site evaluation? Results of a randomized trial. Gastrointest Endosc 2014;80:1056-63. [Crossref] [PubMed]
- Adler DG, Muthusamy VR, Ehrlich DS, et al. A multicenter evaluation of a new EUS core biopsy needle: Experience in 200 patients. Endosc Ultrasound 2019;8:99-104. [Crossref] [PubMed]
- Tian L, Tang A, Zhang L, et al. Evaluation of 22G fine-needle aspiration (FNA) vs. fine-needle biopsy (FNB) for endoscopic ultrasound guided sampling of pancreatic lesions: a prospective comparison study. Surg Endosc 2018;32:3533-9. [Crossref] [PubMed]
- Ayres LR, Kmiotek EK, Lam E, et al. A comparison of endoscopic ultrasound-guided fine-needle aspiration and fine-needle biopsy in the diagnosis of solid pancreatic lesions. Can J Gastroenterol Hepatol 2018;2018:1415062 [Crossref] [PubMed]
- Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy 2016;48:339-49. [PubMed]
- Chrissian A, Misselhorn D, Chen A. Endobronchial-ultrasound guided miniforceps biopsy of mediastinal and hilar lesions. Ann Thorac Surg 2011;92:284-8. [Crossref] [PubMed]
- Cheng G, Mahajan A, Oh S, et al. Endobronchial ultrasound-guided intranodal forceps biopsy (EBUS-IFB) – technical review. J Thorac Dis 2019;11:4049-58. [Crossref] [PubMed]
- Bellinger C, LoVerde D, Haponik E. Performance of different linear endobronchial ultrasound guided transbronchial aspiration needles. Am J Resp Crit Care Med 2017;195:A2878.
- Sharples LD, Jackson C, Wheaton E, et al. Clinical effectiveness and cost-effectiveness of endobronchial and endoscopic ultrasound relative to surgical staging in potentially resectable lung cancer: results from the ASTER randomised controlled trial. Health Technol Assess 2012;16:1-75. [Crossref] [PubMed]
- Grove DA, Bechara RI, Josephs JS, et al. Comparative cost analysis of endobronchial ultrasound-guided and blind TBNA in the evaluation of hilar and mediastinal lymphadenopathy. J Bronchology Interv Pulmonol 2012;19:182-7. [Crossref] [PubMed]
- Chouaid C, Salaun M, Gounant V, et al. Clinical efficacy and cost-effectiveness of endobronchial ultrasound-guided transbronchial needle aspiration for preoperative staging of non-small-cell lung cancer: Results of a French prospective multicenter trial (EVIEPEB). PLoS One 2019;14:e0208992 [Crossref] [PubMed]
- Navani N, Nankivell M, Lawrence DR, et al. Lung cancer diagnosis and staging with endobronchial ultrasound-guided transbronchial needle aspiration compared with conventional approaches: an open-label, pragmatic, randomised controlled trial. Lancet Respir Med 2015;3:282-9. [Crossref] [PubMed]
- Shingyoji M, Nakajima T, Yoshino M, et al. Endobronchial ultrasonography for positron emission tomography and computed tomography-negative lymph node staging in non-small cell lung cancer. Ann Thorac Surg 2014;98:1762-7. [Crossref] [PubMed]
- Ong P, Grosu H, Eapen GA, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for systematic nodal staging of lung cancer in patients with N0 disease by computed tomography and integrated positron emission tomography-computed tomography. Ann Am Thorac Soc 2015;12:415-9. [Crossref] [PubMed]
- Ozturk A, Demirci NY, Aktas Z, et al. EBUS may arise as an initial time saving procedure in patients who are suspected to have small cell lung cancer. Clin Respir J 2018;12:517-23. [Crossref] [PubMed]
- Sampsonas F, Kakoullis L, Lykouras D, et al. EBUS: Faster, cheaper and most effective in lung cancer staging. Int J Clin Pract 2018;72:e13053 [Crossref] [PubMed]
- Warren WA, Sobieszczyk MJ, Sarkar S, et al. Endobronchial ultrasound bronchoscopy: current uses, innovations and future directions. AME Med J 2018;3:70. [Crossref]
- Steinfort DP, Irving LB. Endobronchial ultrasound staging of thyroid lesion in small cell lung carcinoma. Thorac Cardiovasc Surg 2010;58:128-9. [Crossref] [PubMed]
- Casal RF, Phan MN, Keshava K, et al. The use of endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of thyroid lesions. BMC Endocr Disord 2014;14:88. [Crossref] [PubMed]
- Chambers DM, Pfister GJ, Gauhar UA. Linear EBUS-guided fiducial marker placement to guide radiotherapy for endobronchial, radiographically occult synchronous primary squamous cell carcinoma of the lung. Respir Med Case Rep 2017;22:60-3. [Crossref] [PubMed]
- Argento AC, Decker R, Puchalski J. Fiducial marker placement via convex probe EBUS. J Bronchology Interv Pulmonol 2016;23:181-5. [Crossref] [PubMed]
- Khan F, Anker CJ, Garrison G, et al. Endobronchial ultrasound-guided transbronchial needle injection for local control of recurrent non-small cell lung cancer. Ann Am Thorac Soc 2015;12:101-4. [Crossref] [PubMed]
- Kinsey CM. Endobronchial ultrasound-guided transbronchial needle injection for direct therapy of lung cancer. AME Med J 2018;3:74-9. [Crossref]
- Li P, Zheng W, Zhao L. Convex probe endobronchial ultrasound: applications beyond conventional indications. J Thorac Dis 2015;7:E289-97. [PubMed]
- Aumiller J, Herth FJ, Krasnik M, et al. Endobronchial ultrasound for detecting central pulmonary emboli: a pilot study. Respiration 2009;77:298-302. [Crossref] [PubMed]
- Nakajima T, Yasufuku K, Shibuya K, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the treatment of central airway stenosis caused by a mediastinal cyst. Eur J Cardiothorac Surg 2007;32:538-40. [Crossref] [PubMed]
- Casal RF, Jimenez CA, Mehran RJ, et al. Infected mediastinal bronchogenic cyst successfully treated by endobronchial ultrasound-guided fine-needle aspiration. Ann Thorac Surg 2010;90:e52-3. [Crossref] [PubMed]
- Sharma RK, Khanna A, Talwar D. Endobronchial ultrasound: A new technique of pericardiocentesis in posterior loculated pericardial effusion. Chest 2016;150:e121-3. [Crossref] [PubMed]
- Hohenforst-Schmidt W, Zarogoulidis P, Steinheimer M, et al. A new endobronchial ultrasound (EBUS) application for benign and malignant pericardial effusion (PE) aspiration: transbronchial pericardial effusion aspiration (TPEA) with a regular EBUS transbronchial (TBNA) needle under apneic nasal jet-catheter ventilation. J Biomed 2016;1:9-25. [Crossref]
Cite this article as: Yang J, De Cardenas J, Nobari M, Miller R, Cheng G. Narrative review of tools for endoscopic ultrasound-guided biopsy of mediastinal nodes. Mediastinum 2020;4:34.