
Liposarcomas of the mediastinum
Introduction
Liposarcoma is one of the most common tumors to arise from the soft tissues in humans; yet, such tumors originating from the mediastinal compartment are relatively rare. Because of their rarity, most reported cases are single case reports with only few large series recorded in the literature, some of them encompassing tumors arising from all intrathoracic organs including lung, pleura and mediastinum (1-6). It appears from review of the literature that all histologic types of liposarcoma may arise as a primary tumor in the mediastinum. Metastases of liposarcoma to the mediastinum have also been reported but appear to be even less common than primary mediastinal liposarcomas. The latter require a thorough clinical history to distinguish them from primary mediastinal liposarcomas. The World Health Organization (WHO) recognizes four basic types of liposarcoma: well-differentiated/atypical lipomatous tumor (WDL/ALT), dedifferentiated, myxoid and pleomorphic liposarcoma (PLS) (7). All four types of liposarcoma have been recorded as primary tumors in the mediastinum and all have been observed in most mediastinal compartments. In addition, a few unusual variants have also been described in the mediastinum including PLS, myxoid well-differentiated liposarcoma, thymoliposarcoma, and sclerosing high-grade liposarcoma (1-6). In recent years, our understanding of these tumors has exponentially increased as molecular techniques have permitted better delineation of their oncogenic mechanisms. The molecular landscape of liposarcoma is defined primarily by two basic mechanisms of tumorigenesis. Well-differentiated/ALT and dedifferentiated liposarcomas (DDLS) are characterized by supernumerary ring and marker chromosomes that contain amplified sequences of chromosome region 12q13-15, including genes such as MDM2, CDK4 and CPM (8,9). Myxoid liposarcoma (MLS), on the other hand, follows a different oncogenic mechanism and is characterized by a t(12;16)(q13;p11), which leads to the formation of a fusion oncogene: FUS-DDIT3 (DDIT3 was formerly known as CHOP) in 95% of cases or an EWSR1-DDIT3 in about 5% of cases (10-12). PLS, a rare subtype of liposarcoma, is characterized primarily by genomic instability with various complex cytogenetic alterations, although some studies have shown some of these cytogenetic alterations occur more often than others (7). When recurrent alterations are present, they can routinely be detected by cytogenetic and molecular testing including fluorescence in situ hybridization (FISH), array based techniques, polymerase chain reaction (PCR) based assays, and next generation sequencing (NGS) greatly enhancing our capability for diagnosing these tumors. The present review will focus on the clinicopathologic features of the various histologic types of liposarcoma described in the mediastinum and their differential diagnosis. Data is derived from review of the largest series published in the more recent literature on these tumors (1-4).
WDL/ALT
WDL/ALT is a low-grade adipocytic malignancy that most commonly arises in the deep soft tissues of the extremities, inguinal region and retroperitoneum (7). The tumors are characterized by a proliferation of mostly mature adipose tissue admixed with scattered atypical lipoblastic cells. WDL/ALT is the most common type of liposarcoma in the mediastinum. Review of the more recent literature indicates that mediastinal WDL/ALT is more frequent in the anterior compartment than in other areas of the mediastinum (Table 1) (1-4). It more often affects middle aged to elderly adults but is also one of the most common types of mediastinal sarcomas in children (1). The tumors show a slight predilection for men (M/F: 1.3:1). Most tumors seem to arise from the soft tissues in the mediastinum, unrelated to any of the surrounding structures, but tumors clearly originating within the thymus (i.e., primary thymic liposarcoma) have also been rarely documented (1,13). Five cases in the series of anterior mediastinal liposarcomas by Klimstra et al. (1) were said to contain residual involuting thymic tissue adjacent to the tumor, and in two cases thymic tissue could be identified within the tumor. Pericardial adipose tissue is another possible primary site for mediastinal liposarcomas and at least one case has been reported in that location (14). The tumors tend to show an expansile rather than infiltrative growth pattern and may be encapsulated and well-circumscribed. Size may vary from 5 to 40 cm; in the study by Klimstra et al. the mean size was 15.7 cm (1). Some tumors are asymptomatic and discovered incidentally on routine chest X-rays or on CT scans done for other causes; when symptomatic the tumors present with respiratory symptoms due to compression of the lung and airways. The clinical behavior of these tumors is usually characterized by multiple recurrences, with death ensuing after many years due to compromise of local structures (1). Review of the recent literature shows that 20/28 patients with follow-up were alive without evidence of disease between 7–123 months; 4/28 patients were alive with disease between 50–101 months, and 4/28 patients died of their tumors between 11–51 months (Table 1).

Full table
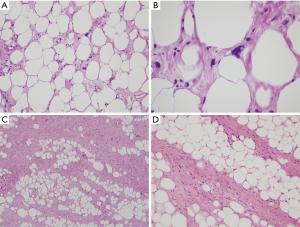
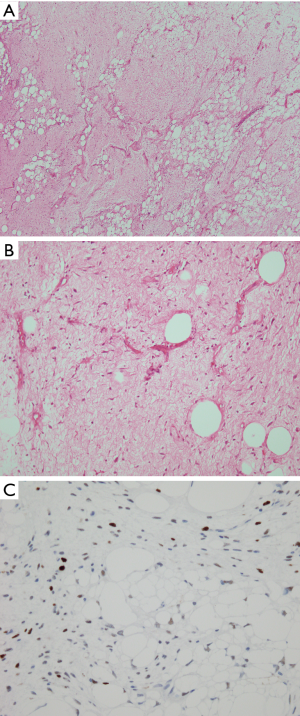
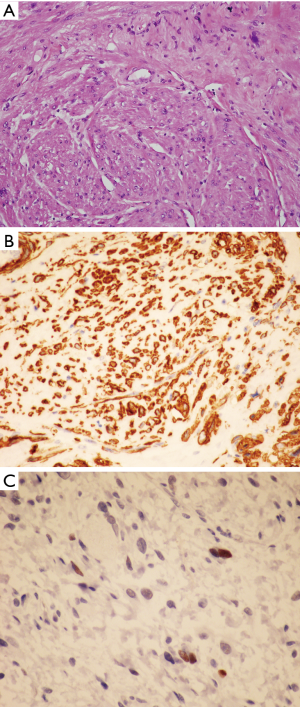
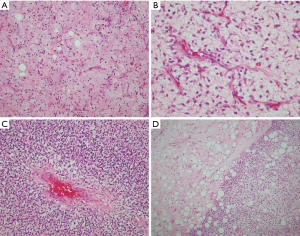
The histologic features of WDL/ALT in the mediastinum recapitulate that of their counterpart in other locations. The majority of the reported tumors are of the “adipocytic” or “lipomatous” variety, characterized by a predominance of mature adipose tissue containing scattered atypical lipoblastic cells (Figure 1A,B). The lipoblasts display large, often hyperchromatic nuclei with multivacuolated cytoplasm; the atypical lipoblastic cells can often be found within fibrous septa in the tumor (1-4,7). Multinucleated and floret-type cells are less commonly encountered. One case reported in the series by Hahn et al. was of the sclerosing type, characterized by abundant collagen stromal deposition harboring multinucleated and mononuclear vacuolated lipoblasts (Figure 1C,D) (2). Six cases in the study by Klimstra et al. showed a prominent inflammatory component with hyperplastic lymphoid follicles that often obscured the neoplastic elements (1). Such tumors can pose a challenge for diagnosis in small biopsy samples in which the better-differentiated adipocytic components may not be readily recognized due to sampling. Three cases in the study by Hahn et al. were of the “spindle cell type”, characterized by bland neural-like spindle cells in a variably myxoid matrix admixed with areas of adipocytic WDL (2). Such cases present a challenge for diagnosis since a variety of soft tissue spindle cell tumors can harbor an adipocytic component despite not being liposarcomas (such as “lipomatous hemangiopericytoma”/lipomatous solitary fibrous tumor); cases with such features require molecular testing to support the diagnosis by demonstrating amplification of MDM2 prior to rendering a diagnosis of WDL. In a study by Sioletic et al., five cases corresponded to a variant of WDL/ALT characterized by an abundance of myxoid stroma, closely resembling MLS (Figure 2A,B) (15). The mimicry of these tumors with MLS can be quite striking, down to the “chicken-wire” pattern of vasculature; however, they almost always contain areas of conventional adipocytic WDL/ALT, a feature that is not expected to occur in true MLS. A simple way to distinguish between the two is by immunohistochemistry; these tumors show scattered nuclear positivity for MDM2, unlike true MLS which is negative for this marker (Figure 2C). Confirmation by FISH (or other assays that can detect copy number alterations and fusions) for MDM2 amplification or DDIT3 rearrangement allows for confident separation of true MLS from this mimic. Four other reported cases in the literature corresponded to another unusual variant of WDL/ALT showing biphenotypic differentiation, in which in addition to the well-differentiated liposarcomatous component, the tumors also contained areas of smooth muscle differentiation (“lipoleiomyosarcoma”) (Figure 3A) (16-18). These cases can also pose a diagnostic challenge on small biopsy samples in which only the smooth muscle component is identified leading to a misdiagnosis of leiomyoma or leiomyosarcoma. When evaluating core biopsies of large masses in the mediastinum or retroperitoneum it is always advisable to include a stain for MDM2 in the panel given that lipoleiomyosarcoma will show nuclear positivity for MDM2 along with the expression of smooth muscle-associated markers, unlike primary smooth muscle tumors which are negative for MDM2 (Figure 3B,C).
The role of immunohistochemistry and molecular pathology has been amply studied in these tumors. Immunostaining for MDM2, CDK4 and p16 is valuable for distinguishing WDL/ALT from atypical lipomas and conventional benign lipomas, which are generally negative for these markers (9,19). Although some studies have found a good correlation between MDM2 immunohistochemistry and amplification of MDM2 by FISH or PCR, false-negative immunohistochemistry does occur (20). In cases in which there is a significant suspicion of malignancy it is always advisable confirm the immunohistochemical findings with cytogenetic or molecular techniques to rule out false negative IHC.
DDLS
DDLS is defined by the WHO as a WDL containing a non-lipogenic sarcomatous component that may be either low or high-grade (7). The most common location for these tumors is the retroperitoneum, followed by the extremities (7). DDLS is the second most common type of liposarcoma of the mediastinum and shows an equal distribution between the anterior and the posterior mediastinal compartment (Table 2) (1-4). The tumors are more common in adults and have an equal gender distribution. Tumor size varies from 9–61 cm, but most are large at initial diagnosis and present with symptoms due to compression of surrounding structures. The behavior of DDLS is generally regarded as intermediate between WDL/ALT and PLS; mediastinal DDLS seem to follow the same rule. In reviewing the largest series (Table 2), 8/12 patients with follow-up were alive without evidence of disease between 6–60 months; 2 patients were alive with disease between 12–72 months, and 1 patient died of disease at 34 months. Unfortunately, the follow-up periods for these cases was short and no long-term follow-up was available; it is thus likely that longer clinical follow-up may disclose similar behavior as in the retroperitoneum where most of these tumors show local recurrences in patients who are followed for more than 10 years. Distant metastases are rare in DDLS and overall mortality has been cited between 28–30% at 5 years (7). A curious finding observed in the retroperitoneum is that the grade of the dedifferentiated component does not appear to influence prognosis and tumors with both low-grade and high-grade dedifferentiated components behave in a similar fashion, although it remains to be determined if the same situation applies to mediastinal tumors. It is also of interest that despite the high-grade morphology, DDLS appear to observe a much more indolent behavior than other sarcomas with comparable high-grade morphology, underscoring the importance for making the correct diagnosis (7).

Full table
The histology of DDLS within the mediastinum can be quite variable depending on the composition of the dedifferentiated component. Eight out of 17 cases from the four largest series summarized in Table 2 showed a dedifferentiated component with low-grade histology; 9 cases were high-grade; and 1 case showed smooth muscle differentiation (lipoleiomyosarcoma). The low-grade dedifferentiated component most often presents as a relatively bland proliferation of fibroblast-like spindle cells with mild nuclear atypia (Figure 4A,B). Two cases also showed metaplastic ossification. One case in Boland’s series and one case in Hahn’s series displayed an unusual but highly distinctive feature characterized by meningothelial-like whorls, a feature that has been previously documented in retroperitoneal locations (Figure 4C) (2,3). The case with smooth muscle differentiation showed a low-grade fascicular proliferation with expression of smooth muscle markers reminiscent of well-differentiated leiomyosarcoma. The high-grade cases showed features reminiscent of undifferentiated pleomorphic sarcoma or high-grade myxofibrosarcoma (Figure 4D). One case in the Boland et al. series contained heterologous osteosarcomatous elements (3). Interestingly, not all cases of DDLS are associated with a low-grade conventional WDL/ALT component, and the diagnosis in such cases requires cytogenetic or molecular confirmation of MDM2 amplification to establish the diagnosis. Of the mediastinal cases summarized in Table 2, three cases in the Boland et al. (3) series were confirmed by FISH, and three cases in the Ortega et al. (4) series had positive immunostaining for MDM2; however none of the cases in the series by Hahn et al. (2) were studied by either FISH or IHC for MDM2.
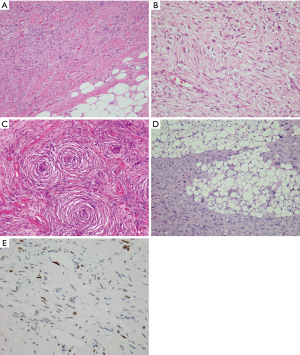
Like WDL, the diagnosis of DDLS is facilitated using antibodies for MDM2, which will show scattered nuclear positivity in the spindle cells of the non-lipogenic component as well as in the neoplastic adipocytic component (Figure 4E). Because the low-grade form of DDLS can have a wide spectrum of morphologic appearances, cases associated with a relatively bland spindle cell proliferation with prominent stromal sclerosis may be confused for solitary fibrous tumor. A caveat to keep in mind in such cases is that STAT6, an immunohistochemical marker that identifies the gene product of the NAB2-STAT6 fusion oncogene (considered to be diagnostic of solitary fibrous tumor) can also be expressed and amplified in a subset of DDLS, including low-grade and high-grade DDLS, a feature that should be kept in mind for the differential diagnosis of these tumors (21).
MLS
MLS is a special variant of liposarcoma characterized by a prominent myxoid matrix. It differs from conventional liposarcomas in that well-differentiated areas composed of mature or normal-appearing adipocytic elements are not a feature of these tumors; instead, the tumor is composed of scattered round to oval-shaped non-lipogenic small cells admixed with a variable number of signet-ring lipoblasts and embedded in abundant myxoid stroma (7). The tumor cells in MLS may also have a vaguely spindled appearance to them (7). The stroma generally displays a very distinctive branching pattern of small vessels that has been likened to a chicken-wire fence. Another distinctive feature of these tumors is that it follows a different oncogenic pathway than WDL/ALT. MLS is characterized by an FUS-DDIT3 or, less commonly, an EWSR1-DDIT3 rearrangement (22,23). Progression of tumorigenesis in these tumors leads to a highly cellular variant that was called in the past “round cell liposarcoma”, but is currently accepted as a high-grade, poorly differentiated variant of the same tumor (7). MLS is one of the least common types of liposarcomas in adults but is quite common in children (24). MLS appears to be the least common type of liposarcoma arising in the mediastinum; in the 4 largest series, 14/90 total cases corresponded to this histotype, although that number may likely be lower given that some of the cases may have corresponded to the myxoid variant of WDL/ALT which had not yet been described at the time of publication of at least 3 of those studies (1-3). The majority of the tumors were located in the anterior mediastinum (9 cases) and 5 cases were in the posterior mediastinum. Gender distribution was approximately equal (4 men, 3 women) and the size tended to be smaller than WDL/ALT and DDLS; although one case in the series by Hahn et al. measured 20 cm (2). Given that none of the cases in their series were studied by immunohistochemistry or FISH, it is possible that the latter case also corresponded to a myxoid variant of WDL/ALT. The clinical behavior of MLS can be quite variable depending on the grade of the tumor, with the higher grades showing a more aggressive behavior. All MLS are associated with metastatic potential. Of the cases reviewed in Table 3, 5 patients were alive with no evidence of disease at 38 months; 1 was alive with disease at 36 months (3 years old child), and 8 were dead of disease between 4–72 months. The two cases reported by Boland et al. suffered multiple recurrences but no metastases; both patients eventually died with disease (3). The two cases reported by Hahn et al. did not show any recurrences or metastases; one patient with low-grade MLS was alive with no evidence of disease at 36 months; the patient with high-grade MLS was lost to follow up (2). Two patients in the study by Ortega et al. showed widespread metastases to lung, pleura, chest wall, ovaries, thyroid, pancreas and pituitary and died between 3 months and 4 years after diagnosis (4).

Full table
The histology of low-grade MLS is characterized by sheets of small round to oval cells with small nuclei, scant cytoplasm and very low mitotic activity (Figure 5A). Scattered signet-ring cell lipoblasts are identified in most cases. The cells are suspended in an abundant myxoid stroma that contains an arborizing network of delicate small branching vessels resembling chicken wire (Figure 5B). Pools of mucin flanked by tumor cells are seen in some cases resembling lymphangioma and are commonly referred to as the “pulmonary edema” pattern. Out of 14 cases reviewed from the recent literature (Table 3), 10 were of low-grade (paucicellular) histology. Four cases were of high-grade histology with a predominant hypercellular, round cell component. The high-grade tumors showed compact sheets of round blue cells with round nuclei containing small nucleoli and scant cytoplasm (Figure 5C). The stroma varied from areas that were myxoid to hypercellular areas devoid of any myxoid stroma; transitions between hypercellular areas composed entirely of small round blue cells and areas that were myxoid could be often encountered (Figure 5D). The differential diagnosis for these tumors includes other forms of small round blue cell sarcomas involving the mediastinum, including embryonal rhabdomyosarcoma, Ewing’s sarcoma and variants, and other small round blue cell tumors such as lymphoma, neuroblastoma and small cell carcinoma. Immunohistochemistry is not of great help for the diagnosis and only serves to rule out some of the alternate possibilities; the low-grade tumors usually show positivity for vimentin and S-100 protein but are negative for all other markers. The most reliable ancillary techniques for diagnosis are cytogenetic or molecular testing with FISH and NGS, which will identify the recurrent FUS-DDIT3 or EWSR1-DDIT3 fusions (22,23).
PLS
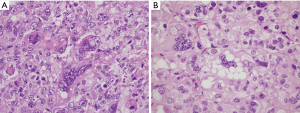
PLS is the rarest type of liposarcoma in the soft tissues and represents a high-grade, poorly differentiated malignancy with generally poor outcome. The tumor has been defined as a high-grade pleomorphic sarcoma that contains a variable number of pleomorphic lipoblasts in the absence of a WDL/ALT component (Figure 6A,B) (7). Tumors with identical features in which WDL/ALT can be identified are currently classified as the “homologous” variant of high-grade DDLS (25-27). The tumors preferentially arise in elderly patients and most cases have been described in the extremities. Mediastinal locations are rare; in the four largest reported series they accounted for 17% of all cases of mediastinal liposarcomas (Table 4), an incidence that is slightly higher than for soft tissue liposarcomas in general (1-4). The tumors occurred in the anterior mediastinum in 8 cases, posterior mediastinum in 4, middle mediastinum in 1, and was multifocal in 1 case. There was an approximately equal gender distribution and they varied in size from 2.2–19 cm. PLS is an aggressive malignancy with high incidence of recurrence and metastases and an overall 5-year survival of 60% (28,29). In the four largest series of mediastinal liposarcomas reviewed, 2 patients were alive with disease at 12 months; 4 were alive with disease from 3–12 months, and 5 died of tumor (survival times not available for most of them). It should be pointed out, however, that most of the cases reported in the mediastinum have had very limited follow-up periods making it difficult to evaluate their true biologic behavior. Difficulty in the diagnosis of PLS stems from the lack of definitive criteria that can lead to variability in the interpretation even amongst bone and soft tissue pathologists. This is borne out in the published literature by the wide variability of the histologic descriptions for these tumors (1-4). The cases presented in the larger published series (Table 4) ranged from tumors with predominance of spindle cells and necrosis, with widespread anaplasia and scattered atypical lipoblasts, to cases resembling myxofibrosarcoma containing a few scattered lipoblasts, to tumors resembling undifferentiated pleomorphic sarcomas with lipoblasts. The series by Boland et al. also included one case of epithelioid PLS (3). Unfortunately, many poorly differentiated malignancies can contain atypical pleomorphic cells with cytoplasmic vacuolation that can closely resemble lipoblasts and this finding may result from radiation-induced changes, degenerative changes, or may be simple mimics, ie so called “lipoblast-like” cells. Determining whether such cells correspond to “atypical/pleomorphic lipoblasts” or simply represent pleomorphic malignant cells with secondary cytoplasmic vacuolation or degenerative changes can be highly arbitrary. This issue is compounded by the fact that there are no distinctive immunohistochemical markers currently associated with these tumors to facilitate the diagnosis. The genetic profile of PLS closely resembles that of other high-grade pleomorphic sarcomas, with complex karyotypes and frequent gains of many chromosomal regions. Although some studies have shown an increased rate of TP53 mutations and deletions of 12q14.2-q14.3 (containing RB1), these changes often occur in the background of exceedingly complex karyotypes that will resemble other poorly differentiated high-grade sarcomas (29-32). PLS does not exhibit amplification of MDM2 or CDK4 and lacks the FUS-DDIT3 or EWSR1-DDIT3 fusion genes, however it generally does not enter the differential of WDL or MLS and may only have to be distinguished from a de-differentiated liposarcoma with high grade features. While identification of RB1 and TP53 alterations may provide some additional information about the differentiation of the tumor, cytogenetic and molecular techniques are less commonly used in routine clinical practice in the diagnosis of PLS.

Full table
Other rare types of mediastinal liposarcomas
A series of unusual variants of liposarcoma that do not fit into any of the previous categories have been described over the years that deserve brief separate mention. These include thymoliposarcoma, lipoleiomyosarcoma, myxoid WDL, epithelioid PLS, pleomorphic MLS, and sclerosing high-grade liposarcoma.
Thymoliposarcoma
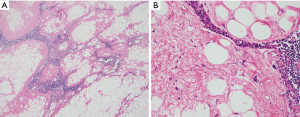
This is a term coined by Havlícek and Rosai in which a WDL appears to be arising within the stromal fatty compartment of the thymus; it was therefore interpreted as a true primary liposarcoma arising from the thymus (Figure 7A,B) (13). A few additional cases have been reported in the literature that were interpreted as originating from thymic stroma, however, none have been studied using modern genetic molecular techniques. So far only three cases fitting the description of Havlícek and Rosai have been reported (1,13). This tumor appears to have a similar natural history as conventional WDL/ALT, with late recurrences and occasional fatal outcome (13).
Lipoleiomyosarcoma
This represents a tumor displaying an unusual biphenotypic pattern of differentiation composed of an admixture within the same neoplasm of WDL/ALT elements and areas showing evidence of smooth muscle differentiation (16-18). A few rare cases have been described in addition to the few cases presented in Table 1. Gómez-Román et al. and Folpe et al. reported one case each in the mediastinum showing these features (18,33). More recently Weissferdt et al. reported 3 cases of lipomatous mediastinal tumors with muscle differentiation, two of which were liposarcomas and one a thymolipoma (34). The myogenic differentiation can be of either smooth or skeletal muscle type in these tumors (35,36). Myogenic differentiation can occur in both WDL/ALT and in DDLS. In our study of liposarcomas of the posterior mediastinum, one case of DDLS was of lipoleiomyosarcoma type; MDM2 immunohistochemistry was positive in both the conventional WDL/ALT component and in the smooth muscle component (4). The smooth muscle component in such tumors is generally well-differentiated with minimal cytologic atypia and mitotic activity. The biologic behavior is similar to other DDLS (18). Given that the dedifferentiated components of DDLS can recapitulate numerous other tissue types including fibrous tissue, smooth muscle, and neural tissue as well as heterologous elements like bone and cartilage, it is possible that this tumor type simply represents a DDLS with a “differentiated”-dedifferentiated component resembling smooth muscle.
Myxoid WDL
This is a relatively recently described variant of WDL/ALT that is characterized by prominent areas displaying abundant myxoid changes with a chicken-wire pattern of stromal vessels that can be easily mistaken for low-grade MLS (15). In the four largest series, 5 cases showed features consistent with this tumor, although it is likely that some of the cases included under the diagnosis of “myxoid liposarcoma” in some of the older studies might have corresponded to this variant of WDL/ALT (3,36,37). This tumor represents a common pitfall for diagnosis and is likely to be more common than is presently recognized. The diagnosis is made by demonstrating MDM2 immunostaining of the tumor cells or amplification of MDM2 by FISH, an event that does not occur in true MLS (15). Another clue to the diagnosis is the presence of areas of conventional WDL/ALT admixed and transitioning with the myxoid areas, a finding that is not observed in true MLS.
Epithelioid PLS
This is an extremely rare variant of liposarcoma described by Miettinen and Enzinger that is characterized by sheets of epithelioid cells with only occasional atypical scattered lipoblastic cells present, and which can simulate a metastasis from an epithelial malignancy (38). The tumor cells can have large lipid filled vacuoles and can also show focally signet-ring cell morphology. The resemblance with carcinoma is enhanced due to reactivity for cytokeratin antibodies in up to 50% of cases (38). At least 3 cases have been described in mediastinal location; one of the patients for whom clinical follow-up was available died of disease at 2 months post excision (3,39,40). A cytogenetic study using FISH by Wang et al. demonstrated absence of amplification for MDM2 in this variant of liposarcoma in a large series of these tumors (41). Identification of scattered lipoblasts is indispensable for the diagnosis.
Pleomorphic MLS
This is a designation proposed by Alaggio et al. for tumors preferentially arising in teenagers and young adults that are characterized by hypocellular myxoid areas that mimic MLS admixed with areas of more typical PLS (42). Such tumors seem to have a predilection for the mediastinum in young patients and are associated with a poor prognosis (3). Although these tumors were initially considered to be a variant of MLS, evidence of DDIT3 rearrangements have not yet been demonstrated (3). Making the distinction between this tumor and a conventional PLS with myxoid stroma or myxofibrosarcomatous features can often be arbitrary, the only distinguishing argument being that pleomorphic MLS is more common in children and young adults. The prognosis is very similar to PLS in adults.
Sclerosing high-grade liposarcoma
This is a very unusual variant of PLS described by Suster and Morrison that is characterized by extensive stromal sclerosis containing numerous scattered atypical multivacuolated lipoblasts (43). The study included 8 cases presenting in the retroperitoneum, retropubic space, arm and spermatic cord in 4 men and 4 women aged 39–90 years (43). Although no cases were identified in the mediastinum, we have since seen a case arising within the mediastinum of a 65-year-old woman; the tumor showed extensive stromal sclerosis with numerous singly scattered atypical lipoblastic cells with multiple cytoplasmic vacuoles; areas of more conventional PLS composed of a cellular pleomorphic high grade sarcoma with scattered lipoblasts were also present in some sections. In the original study, 4 cases arose de novo and 4 represented recurrences of tumors that had been previously diagnosed as conventional liposarcoma (41). It was postulated that the tumors may represent an end-stage pathway for various types of liposarcomas.
Summary
Mediastinal liposarcomas comprise a heterogeneous group of tumors with diverse morphology that have the potential for highly aggressive behavior and patient death caused by tumor. Correct diagnosis is of importance for appropriate choice of therapy. Immunohistochemistry can be of assistance in WDL/ALT and DDLS with the use of antibodies for MDM2, although confirmation of amplification of MDM2 is always advisable in equivocal cases. The myxoid variant of liposarcoma may be harder to identify in some instances, particularly the hypercellular variant, and may require molecular testing to demonstrate the DDIT3 gene rearrangement. PLS remains an elusive diagnosis that may occasionally be arbitrarily applied due to the lack of distinct a molecular signature or immunohistochemical profile, and which will require further studies to better delineate its diagnostic criteria. A significant number of unusual variants or less common variants have also been described which require awareness for proper identification.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Mediastinum for the series “Mediastinal Sarcomas”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-42). The series “Mediastinal Sarcomas” was commissioned by the editorial office without any funding or sponsorship. DIS and SS served as the unpaid Guest Editors of the series. SS serves as an unpaid editorial board member of Mediastinum from June 2019 to May 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Klimstra DS, Moran CA, Perino G, et al. Liposarcoma of the anterior mediastinum. A clinicopathologic study of 28 cases. Am J Surg Pathol 1995;19:782-91. [Crossref] [PubMed]
- Hahn HP, Fletcher CDM. Primary mediastinal liposarcoma. Clinicopathologic analysis of 24 cases. Am J Surg Pathol 2007;31:1868-74. [Crossref] [PubMed]
- Boland JM, Colby TV, Folpe AL. Liposarcomas of the mediastinum and thorax. A clinicopathologic and molecular cytogenetic study of 24 cases, emphasizing unusual and diverse histologic features. Am J Surg Pathol 2012;36:1395-403. [Crossref] [PubMed]
- Ortega P, Suster D, Falconieri G, et al. Liposarcomas of the posterior mediastinum: clinicopathologic study of 18 cases. Mod Pathol 2015;28:721-31. [Crossref] [PubMed]
- Chen M, Yang J, Zhu L, et al. Primary intrathoracic liposarcoma: a clinicopathologic study and prognostic analysis of 23 cases. J Cardiothorac Surg 2014;9:119. [Crossref] [PubMed]
- Fu Z, Yang K, Yang X, et al. Primary intrathoracic liposarcoma: a clinical analysis of 31 cases. Cancer Commun (Lond) 2019;39:15. [Crossref] [PubMed]
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. WHO Classification of Tumors of Soft Tissue and Bone. 4th Edition, IARC. Lyon, 2013:33-43.
- Pedeutour F, Suijkerbuijk RF, Van Gaal J, et al. Chromosome 12 origin in rings and giant markers in well-differentiated liposarcoma. Cancer Genet Cytogenet 1993;66:133-4. [Crossref] [PubMed]
- Sirvent N, Coindre JM, Maire G, et al. Detection of MDM2-CDK4 amplification by fluorescent in-situ hybridization in 200 paraffin-embedded tumor samples: utility in diagnosing adipocytic lesions and comparison with immunohistochemistry and real-time PCR. Am J Surg Pathol 2007;31:1476-89. [Crossref] [PubMed]
- Crozat A, Aman P, Mandahl N, et al. Fusion of CHOP to a novel RNA-binding protein in human myxoid liposarcoma. Nature 1993;363:640-4. [Crossref] [PubMed]
- Turc-Carel C, Limon J, Dal Cin P, et al. Cytogenetic studies of adipose tissue tumors. II. Recurrent reciprocal translocations t(12;16)(q13:p11) in myxoid liposarcomas. Cancer Genet Cytogenet 1986;23:291-9. [Crossref] [PubMed]
- Dal Cin P, Sciot R, Panagopoulous I, et al. Additional evidence of a variant translocation t(12;22) with EWS/CHOP fusion in myxoid liposarcoma: clinicopathological features. J Pathol 1997;182:437-41. [Crossref] [PubMed]
- Havlícek F, Rosai J. A sarcoma of thymic stroma with features of liposarcoma. Am J Clin Pathol 1984;82:217-24. [Crossref] [PubMed]
- Lacey CJN, Petch MC. Primary liposarcoma of the pericardium. Thorax 1979;34:120-2. [Crossref] [PubMed]
- Sioletic S, Dal Cin P, Fletcher CDM, et al. Well-differentiated and dedifferentiated liposarcomas with prominent myxoid stroma: analysis of 56 cases. Histopathology 2013;62:287-93. [Crossref] [PubMed]
- Evans HL. Smooth muscle in atypical lipomatous tumors: a report of three cases. Am J Surg Pathol 1990;14:714-8. [Crossref] [PubMed]
- Suster S, Wong TY, Moran CA. Sarcomas with combined features of liposarcoma and leiomyosarcoma. Study of two cases of an unusual soft tissue tumor showing dual lineage differentiation. Am J Surg Pathol 1993;17:905-11. [Crossref] [PubMed]
- Folpe AL, Weiss SW. Lipoleiomyosarcoma (well-differentiated liposarcoma with leiomyosarcomatous differentiation): a clinicopathologic study of nine cases including one with dedifferentiation. Am J Surg Pathol 2002;26:742-9. [Crossref] [PubMed]
- Thway K, Flora R, Shah C, et al. Diagnostic Utility of p16, CDK4, and MDM2 as an Immunohistochemical Panel in Distinguishing Well-Differentiated and Dedifferentiated Liposarcomas From Other Adipocytic. Tumors. Am J Surg Pathol 2012;36:462-9. [Crossref] [PubMed]
- Thway K, Wang J, Swansbury J, et al. Fluorescence In Situ Hybridization for MDM2 Amplification as a Routine Ancillary Diagnostic Tool for Suspected Well-Differentiated and Dedifferentiated Liposarcomas: Experience at a Tertiary Center. Sarcoma. 2015;2015:812089 [Crossref] [PubMed]
- Doyle LA, Tao D, Mariño-Enriquez A. STAT6 is amplified in a subset of dedifferentiated liposarcoma. Mod Pathol 2014;27:1231-7. [Crossref] [PubMed]
- Rabbitts TH, Forster A, Larson C, et al. Fusion of the dominant negative transcription regulator CHOP with a novel gene FUS by translocation t(12;16) in malignant liposarcoma. Nat Genet 1993;4:175-80. [Crossref] [PubMed]
- Panagopoulos I, Hoglund M, Mertens F. Fusion of the EWS and CHOP genes in myxoid liposarcoma. Oncogene 1996;12:489-94. [PubMed]
- Huh WW, Yuen C, Munsell M, et al. Liposarcoma in children and young adults: a multi-institutional experience. Pediatr Blood Cancer 2011;57:1142-6. [Crossref] [PubMed]
- Boland JM, Weiss SW, Olivera AM, et al. Liposarcomas with mixed well-differentiated and pleomorphic features: a clinicopathologic study of 12 cases. Am J Surg Pathol 2010;34:837-43. [Crossref] [PubMed]
- Henricks WH, Chu YC, Goldblum JR, et al. Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Pathol 1997;21:271-81. [Crossref] [PubMed]
- Mariño-Enríquez A, Fletcher CDM, Dal Cin P, et al. Dedifferentiated liposarcoma with “homologous” lipoblastic (pleomorphic liposarcoma) differentiation: clinicopathologic and molecular analysis of a series suggesting revised diagnostic criteria. Am J Surg Pathol 2010;34:1122-31. [Crossref] [PubMed]
- Gebhard S, Coindre JM, Michels JJ, et al. Pleomorphic liposarcoma: clinicopathologic, immunohistochemical and follow-up analysis of 63 cases: a study from the French Federation of Cancer Centers Sarcoma Group. Am J Surg Pathol 2002;26:601-16. [Crossref] [PubMed]
- Hornick JL, Bosenberg MW, Mentzel T, et al. Pleomorphic liposarcoma: clinicopathologic analysis of 57 cases. Am J Surg Pathol 2004;28:1257-67. [Crossref] [PubMed]
- Fritz B, Schubert F, Wrobel G, et al. Microarray-based copy number and expression profiling in dedifferentiated and pleomorphic liposarcoma. Cancer Res 2002;62:2993-8. [PubMed]
- Idbaih A, Coindre JM, Derre J, et al. Myxoid malignant fibrous histiocytoma and pleomorphic liposarcoma share very similar genomic imbalances. Lab Invest 2005;85:176-81. [Crossref] [PubMed]
- Rieker RJ, Joos S, Bartsch C, et al. Distinct chromosomal imbalances in pleomorphic and in high-grade dedifferentiated liposarcoma. Int J Cancer 2002;99:68-73. [Crossref] [PubMed]
- Gómez-Román JJ, Val-Bernal JF. Lipoleiomyosarcoma of the mediastinum. Pathology 1997;29:428-30. [Crossref] [PubMed]
- Weissferdt A, Moran CA. Lipomatous tumors of the anterior mediastinum with muscle differentiation: a clinicopathological and immunohistochemical study of three cases. Virchows Arch 2014;464:489-93. [Crossref] [PubMed]
- Tallini G, Erlandson RA, Brennan MF, et al. Divergent myosarcomatous differentiation in retroperitoneal liposarcoma. Am J Surg Pathol 1993;17:546-56. [Crossref] [PubMed]
- Schweitzer DL, Aguam AS. Primary liposarcoma of the mediastinum. Report of a case and review of the literature. J Thorac Cardiovasc Surg 1977;74:83-97. [Crossref] [PubMed]
- Standerfer RJ, Armistead SH, Paneth M. Liposarcoma of the mediastinum: report of two cases and review of the literature. Thorax 1981;36:693-4. [Crossref] [PubMed]
- Miettinen M, Enzinger FM. Epithelioid variant of pleomorphic liposarcoma: a study of 12 cases of a distinctive variant of high-grade liposarcoma. Mod Pathol 1999;12:722-8. [PubMed]
- Downes KA, Goldblum JR, Montgomery EA, et al. Pleomorphic liposarcoma: A clinicopathologic analysis of 19 cases. Mod Pathol 2001;14:179-84. [Crossref] [PubMed]
- Huang HY, Antonescu CR. Epithelioid variant of pleomorphic liposarcoma. Immunohistochemical and ultrastructural analysis of six cases with emphasis on overlapping features with epithelioid malignancies. Ultrastruct Pathol 2002;26:299-308. [Crossref] [PubMed]
- Wang L, Ren W, Zhou X, et al. Pleomorphic liposarcoma: a clinicopathological, immunohistochemical and molecular cytogenetic study of 32 additional cases. Pathol Int. 2013;63:523-31. [Crossref] [PubMed]
- Alaggio R, Coffin CM, Weiss SW, et al. Liposarcomas in young patients: a study of 82 cases occurring in patients younger than 22 years of age. Am J Surg Pathol 2009;33:645-58. [Crossref] [PubMed]
- Suster S, Morrison C. Sclerosing poorly differentiated liposarcoma. Clinicopathologic, immunohistochemical and molecular analysis of a distinct subtype of lipomatous tumor of soft tissue. Histopathology 2008;52:283-93. [Crossref] [PubMed]
Cite this article as: Suster DI, Suster S. Liposarcomas of the mediastinum. Mediastinum 2020;4:27.


