
Fibroblastic sarcomas of the mediastinum
Introduction
Primary sarcomas of the mediastinum are rare, accounting for only 1.4% of soft tissue sarcomas (1) and constitute 2% to 8% of primary mediastinal malignancies (2,3). Patients may have symptoms and ominous signs due to the location of these tumors adjacent to vital structures. Almost all entities arising from peripheral soft tissues can be encountered in the mediastinum except for organ-specific tumors, such as gastrointestinal stromal tumors. This article will be focused on some fibroblastic sarcomas of the mediastinum, including solitary fibrous tumor (SFT) (benign and malignant), low grade fibromyxoid sarcoma (LGFMS), and adult fibrosarcoma (FS). Infantile FS is a distinct entity with different clinicopathological and genetic findings and beyond the scope of this article. Sclerosing epithelioid sarcoma and myxofibrosarcoma are extremely rare in the mediastinum and will not be discussed here.
SFT
SFT is usually a well-circumscribed fibroblastic tumor consisting of an admixture of spindle cells and thick collagen stroma with at least focal areas of branching staghorn blood vessels. It was originally thought to be a neoplasm of pleura, other serosal surfaces, and mediastinum. However, it is now recognized as a tumor that commonly affects deep soft tissue and can virtually occur at any location including the meninges (4-7). SFTs can arise in the anterior and posterior mediastinal connective tissue, thymic tissue, pericardium, heart, etc. (6,8-16) without any involvement of the pleura. SFT commonly occur in the anterosuperior and posterior mediastinum and rarely in the middle mediastinum. Most patients are asymptomatic and mediastinal masses are detected incidentally on an imaging study performed for an unrelated condition. Symptomatic patients commonly present with cough, chest pain, dyspnea and other discomfort. Extrathoracic findings associated with mediastinal SFT include clubbing, osteoarthropathy, and hypoglycemia (caused by an insulin-like growth factor produced by the tumor cells). These manifestations are more likely to be seen in patients with large mediastinal masses.
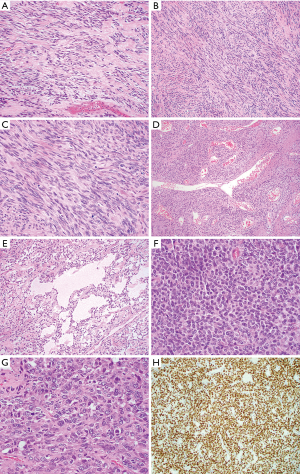
Mediastinal SFTs are rare and the exact incidence is unknown. Review of two larger case series (6,16) and single case reports (summarized in Table 1) together (total 34 cases excluding one case without designating sex in Witkin et al. series) shows a wide age range (27 to 81 years) with a peak of incidence in the fifth and sixth decades with male predominance (a male to female ratio of 2:1). The tumors range from 3.0 to 24.0 cm in size and are usually encapsulated firm masses with lobulated and whorled appearance. The cut surface is white to gray, often associated with focal cystic degeneration. Microscopic examination (Figure 1) shows spindle cell proliferation with areas of hypocellularity and hypercellularity, dense collagenization, and myxoid change. Tumor cells have relatively bland nuclear features with indistinct cell borders and are randomly arranged in strands between thick and keloid-like collagen bundles. A hemangiopericytoma-like blood vessel pattern can be present at least focally in most cases. Mitoses are scarce, usually between 1 and 3 per 10 high power fields. Fat-forming variant of mediastinal SFT have also been described (12). Malignant SFTs are usually characterized by hypercellularity, increased mitotic activity (≥4 mitoses/10 hpf), nuclear atypia and necrosis (17). Malignant lesions tend to be larger and show infiltrative growth borders. The cells of SFT are positive for CD34 (90% to 95% cases) and CD99 (70% cases) and variably positive for EMA, Bcl-2, and SMA (20% to 35% cases). Recent study demonstrated nuclear expression of STAT6 in 98% of 60 SFTs (40 benign, 5 atypical and 15 malignant) with polyclonal antibody (18) and 100% of 54 and 45 SFTs respectively using monoclonal antibody (19,20). STAT6 is a useful diagnostic marker to distinguish SFT from other spindle cell neoplasm or histologic mimics. SFTs harbor a recurrent intrachromosomal fusion between the NAB2 and STAT6 genes on chromosome 12 (NAB2-STAT6) (21-23), which can be detected by RT-PCR. However, the close proximity of these genes makes the fusion difficult to be detected by conventional chromosomal banding or fluorescence in situ hybridization (FISH) techniques. The diagnosis of conventional SFTs is straightforward in well-sampled materials. Diagnostic challenges more often occur in fat-forming and giant cell-rich variants of SFTs and malignant SFTs. STAT6 immunostaining and RT-PCR of NAB2-STAT6 fusion gene should be helpful for rendering a diagnosis of SFT (24).
Full table
Complete surgical excision with negative margins is the treatment choice for both benign and malignant SFT. Limited data that is available suggests that mediastinal SFTs are more aggressive than SFTs of the pleura (6,25), but larger studies are necessary. Aggressive or malignant behavior generally is characterized by local recurrence, intrathoracic spread, and distant metastases. Recurrence can occur years later after the initial excision highlighting the necessity of long-term follow-up. Although long-term follow-up of mediastinal SFT is rarely reported, pleuropulmonary SFT generally shows a benign clinical course, but a subset will recur or metastasize, with an estimated 5-year progression rate in the range of 8.5% (26). Morphological features are not always reliable for predicting tumor behavior, but most histologically benign SFTs do not recur or metastasize after complete excision. Malignant SFT shows a higher metastatic potential than predominantly fibrous SFT (20% to 30% vs. 5%) (27,28). Recently, a proposed risk stratification model using age (≥55 years), size (≥15 cm), mitotic index (≥4 per 10 HPF) and necrosis (≥10%) was developed to predict a high risk of metastasis (29). Molecular predictors of behavior for SFT remain to be identified.
LGFMS
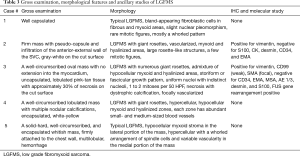
LGFMS is a malignant fibroblastic tumor with deceptively benign spindle cell proliferation, contrasting fibrous and myxoid areas and late metastasis. Hyalinizing spindle cell tumor (HSCT) with giant rosettes is now considered a histological variant of LGFMS as they both share the same genetic abnormalities (30-34). LGFMS typically involves the proximal extremities and trunk. It was first reported by Evans in 1987, who described two female patients with lung metastases. Both patients had histories of resections of bland-appearing soft tissue tumors located in the soft tissues of the scapular area and the axillary–chest wall area, respectively (30). Later, Evans expanded his original series with up to 33 cases published in two series (31,32), that showed the typical histological findings and other unusual features. The exact incidence of LGFMS is unknown because these have been misdiagnosed as other mesenchymal tumors including benign fibrous tissue, desmoid-type fibromatosis, peripheral nerve sheath tumor, monophasic synovial sarcoma, and gastrointestinal stromal tumors (35).
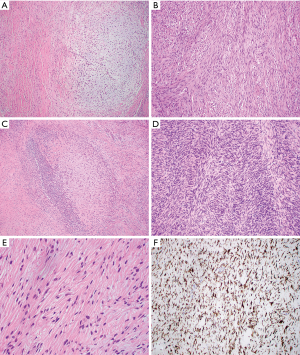
Only rare cases of primary mediastinal LGFMS have been reported in the English literature (36-39) and occurred in the anterior mediastinum (3 cases), the superior mediastinum (1) and the epicardium/right heart (1) (Table 2). The age of patients ranged from 19 to 50 years with male to female ratio 3:1. Tumor size ranged from 7 to 20 cm. Gross examination showed a well circumscribed firm mass with lobular appearance (3 cases), necrosis (1 case), calcification (1 case) and hemorrhage (1 case). The tumor involved the anterior portion of the superior vena cava (1 case), the right heart epicardium (1 case), and chest wall (1 case). Histologically, all these cases displayed areas of characteristic hypocellular myxoid and hyalinized zones (Figure 2 and Table 3). Three cases had giant rosettes (collagenous center surrounded by a cuff of tumor cells). The bland spindle cells were arranged in a storiform or fascicular pattern with rare mitoses and slight nuclear pleomorphism. Vasculature is either focally or diffusely present. Lesional cells are positive for vimentin (diffuse) and SMA (focal) and negative for S100, CK, desmin, CD34, and EMA. The diagnosis can be confirmed by FISH with FUS dual-color break-apart probes or by EM, which shows numerous intermediate filaments and dilated rough endoplasmic reticulum in the spindled cells, indicating the fibroblastic origin. More recently, diffuse and strong cytoplasmic staining for MUC4 has been demonstrated to be a highly sensitive and specific immunohistochemical marker of genetically confirmed LGFMS (40,41). In these studies, all the histologic mimickers of LGFMS were negative for MUC4, except for 30% of monophasic synovial sarcomas analyzed. Some authors consider MUC4 expression, even without confirmatory FISH studies, as a sufficient ancillary test for the diagnosis of LGFMS. The majority of LGFMS harbor a common t(7;16)(q34; p11) resulting in the fusion gene FUS-CREB3L2 while a minority of cases contain t(11;16)(p11;p11) resulting the fusion gene FUS-CREB3L1 (34).
Full table
Full table
All the mediastinal LGFMS cases are essentially identical to their counterparts in the extremities and trunk (32,34). The natural history of LGFMS in the mediastinum also appears to be similar to that of their counterparts elsewhere, with potential for late (greater than 5 years) local recurrence (Table 2). Distant metastases have not yet been reported; this could be due to limited case number and a short period of follow-up. However, the potential for late metastatic spread to the lung and pleura is high, necessitating long-term follow-up for all patients with LGFMS (32,34). Some cases reported outside the mediastinum have unusual cytomorphological features and growth patterns such as hypercellular areas with moderate nuclear pleomorphism, round cell morphology, increased mitoses, and fascicular-herringbone pattern (Figure 2D). Areas of sclerosing epithelioid FS and dedifferentiation with predominantly round cells and numerous mitotic figures have been reported (32). Although dedifferentiation resulted in short survival, unusual morphological features did not have effect on tumor behavior or prognosis. Another large study of 73 cases also showed LGFMS with occasional presence of intermediate- to high-grade sarcoma, which did not have a worse outcome in short term follow-up (33).
Adult FS
Adult FS (or FS) was described as a “malignant tumor, composed of fibroblasts with variable collagen and, in classical cases, a herringbone architecture” by the World Health Organization (WHO) (42). Although it was once considered as the most common adult sarcoma, changes in the diagnostic criteria have made it a rare entity. There is considerable doubt about the veracity of demographic and clinicopathologic features of FS in the older literature without immunostaining and electron microscopy studies (43). Proper classification of soft tissue tumors with morphological features, new immunostaining markers and characteristic molecular changes has resulted in marked decline in the number of adult FS because numerous cases formerly diagnosed as FSs were actually dedifferentiated liposarcoma (DDL), fibromatosis, fibrosarcomatous DFSP, LGFMS, malignant peripheral nerve sheath tumor (MPNST), synovial sarcoma, etc. (43-45). A 2010 review for adult-type FS from a single institution over a 48-year period by Bahrami and Folpe revealed that true adult FS accounts for <1% of approximately 10,000 adult soft tissue sarcomas diagnosed from 1960 to 2008 at Mayo clinic (43).
Mediastinal adult FS is an extremely rare tumor. Only 26 cases of 163 cases previously diagnosed as adult FS over a 48-year period met WHO diagnostic criteria; one case possibly arose from the mediastinum (43). Another large study of 39 low grade FS cases (not otherwise specified) published in 2006 revealed that 6 tumors involved the thorax without mentioning the mediastinum specifically (44). Review of the older English literature from 1955 to 1991 show 7 reported mediastinal FS cases (46-48); it is unclear how many of these would meet the modern definition (48). However, one case of mediastinal FS was confirmed by morphological features and electron microscopy (48). The 34-year-old white female developed chest pain with cough, dyspnea, and dysphagia. Imaging revealed a bulky mass (19.0 cm) in the anterior mediastinum. Grossly, it was well-circumscribed and encapsulated with grey-white appearance on the cut surface. Histological examination revealed cellular, intersecting fascicles composed of plump to spindle cells with a moderate degree of atypia and numerous mitotic figures. Electron microscopy displayed loosely arranged fibroblast-like cells with prominent endoplasmic reticulum and varying amount of collagen fibers around the cells (48).
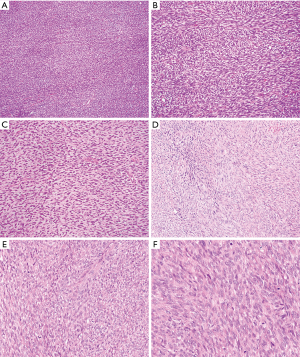
Due to the rarity of mediastinal adult FS, review of this neoplasm must be extrapolated from studies reported at other body sites (43-45). FS most often arises from the deep soft tissues of the extremities, truck, head and neck. It is uncommon in the visceral organs and retroperitoneum, where sarcomatoid carcinoma and low grade dedifferentiated liposarcoma should be ruled out respectively. Adult FS most commonly occurs in middle-aged and older adults (median age, 50 years), with a slight male predominance. Histological examination of adult FS reveals relatively monophasic spindle cells with no more than a moderate degree of pleomorphism (Figure 3). The lesional cells resemble normal fibroblasts and are arranged in fascicles that intersect each other at acute angles, resulting in a herringbone pattern and at times a storiform growth pattern. Lesional cells have elongated hyperchromatic nuclei, variably prominent nucleoli and scanty cytoplasm with variable mitotic activity. The amount of stromal collagen can range from a delicate intercellular network to areas with keloid like sclerosis or hyalinization. Fibromatosis-like areas may be present in some FS. Immunohistochemically, the prototypical FS should be positive for vimentin and type I collagen. The presence of focal immunoreactivity for SMA and laminin indicate myofibroblastic differentiation.
FSs are aggressive. The survival rate in one large series was 41% at 5 years and 29% at 10 years (49). Increased mitoses and hypercellularity were associated with an increased incidence of metastases (49). Another series study of strictly defined adult FS disclosed that at least 80% of tumors were high-grade (FNCLCC grade 2 or 3). One of the four low-grade lesions progressed to a high-grade sarcoma when it recurred. Multiple local recurrences, lymph node and parenchymal metastasis occurred. The overall survival rate was <70% at 2 years and <55% at 5 years. Due to the limited number of FS cases, no correlation could be made between clinicopathological features (such as grading, tumor size) and prognosis (43).
Differential diagnoses
A wide range of spindle cell mesenchymal neoplasms can occur in the mediastinum. The main differential diagnoses for mediastinal SFT include spindle cell thymoma, desmoplastic mesothelioma, monophasic synovial sarcoma, spindle cell lipoma and LGFMS. Spindle cell thymomas are composed of relatively benign spindle to ovoid epithelial cell nests separated by bands of collagen. Other features include lack of mitotic figures and the presence of small immature T lymphocytes (50). Tumor cells are uniformly positive for cytokeratin and P63 (strong and diffuse) and negative for STAT6. Desmoplastic mesothelioma commonly arises from the pleura and consists of atypical cells in a storiform or nonspecific pattern with a dense collagenous background. Atypical cells are positive for CK5/6, WT1, D2-40 and calretinin. Monophasic synovial sarcoma can mimic cellular SFT (previously called hemangiopericytoma) and malignant SFT. They both can display HPC-like or staghorn vascular patterns. Monophasic synovial sarcoma is composed of relatively uniform spindle cells with a high N/C ratio, oval and vesicular nuclei, scant cytoplasm and indistinct cell borders. The tumor cells grow as sheets or fascicles with hyalinized or wiry collagen in the background (50). The mitotic rate is highly variable and dystrophic calcifications are relatively common. The spindle cells are positive for TLE1 (diffuse to patchy), cytokeratin (focal), EMA (focal), and CD99. Majority of synovial sarcoma contain a characteristic t(X;18) balanced translocation, which can be detected by FISH of SYT (SS18) gene or RT-PCR of the SS18-SSX fusion gene. Spindle cell lipoma can also be a diagnostic pitfall for fat-forming SFT. It is typically composed of short stubby spindle cells, distinctive ropy collagen, and a variable adipose component with lack of HPC-like blood vessels. It is usually strongly and diffusely positive for CD34 and negative for STAT6 (18). SFT can be confused with LGFMS, particularly on core biopsies, as both show a benign-looking spindle cell proliferation with collagen in the background. LGFMS is diffusely positive for MUC4 and almost never expresses CD34 or STAT6 (40).
It is important to distinguish LGFMS from benign neoplasms due to LGFMS late metastatic spread. LGFMS can shows hypocellular areas of bland spindle cell morphology with abundant collagenized stroma. The main differential diagnosis of LGFMS includes typical SFT, desmoid fibromatosis, neurofibroma, and MPNST in the mediastinum. Fibrous forms of SFT are composed of variable bland oval spindle cells with no specific growth pattern and variable hyalinized stromal collagen. The stroma may have a variable degree of myxoid change. These features can overlap with LGFMS. However, staghorn blood vessels and medium-sized round vessels with variable perivascular hyalinization are a common feature for SFT (7). Instead LGFMS have curvilinear capillary vessels or arcades of blood vessels, occasionally with perivascular hyalinization in the myxoid areas (34). A subset of LGFMS can have areas of a fascicular growth pattern reminiscent of desmoid fibromatosis (34). Fibromatosis can occur in the mediastinum (51). It has infiltrative borders, but entirely lacks metastatic potential. It displays long fascicles of uniform bland myofibroblastic cells admixed with well-formed medium-sized blood vessels with muscular wall. It does not have the curvilinear blood vessels commonly present in the myxoid areas of LGFMS. Between 70% to 80% of desmoid fibromatosis shows nuclear staining of beta-catenin. LGFMS with focal myxoid change and lack of alternating fibrous and myxoid areas can resemble neurofibroma. Neurofibromas occur in association with nerves in the paraspinal region and present as mass forming lesions in the posterior mediastinum (52). Both show spindle cell proliferation with whorled or vaguely whorled growth pattern and variably myxoid or collagenous stroma. However, neurofibroma is composed of elongated spindle cells with wavy or buckled hyperchromatic nuclei, admixed with a population of short spindle cells. LGFMS consists of bland, short spindle cells with ovoid nuclei and fine chromatin. Heterogeneous cells of neurofibroma are highlighted by S100 (Schwann cells) and CD34 (fibroblasts). MPNST is often associated with a large nerve at the posterior mediastinum and typically show a more fascicular growth pattern than LGFMS with perivascular hypercellularity, tapering nuclei, and rare focal expression of S100.
It is not uncommon that monophasic SS, cellular SFT and MPNST were misdiagnosed as adult FS. As mentioned earlier, only 26 cases (16%) of the 163 putative FSs reported by Bahrami and Folpe met diagnostic criteria for FS. Some previously diagnosed FS cases were reclassified as synovial sarcoma (21 cases, 13%), SFT (14 cases, 9%) and MPNST (8 cases, 5%) (43). This is not surprising because some monophasic SSs consists of tight intersecting fascicles with a herringbone (FS-like) appearance; of note it should be remembered that monophasic SS was not recognized as a definite entity until the mid-1980s (45). However, the presence of HPC-like blood vessels, wiry collagen, stromal calcification and mast cells should always raise consideration of SS. Molecular studies of t(X;18)(p11;q11) should help to confirm or rule out SS. TLE1 immunostaining is not specific, but absence of TLE1 staining helps to rule out SS. Cellular SFT has a monotonous appearance with moderate to high cellularity, round to oval nuclei and little intervening fibrosis, resembling FS and monophasic SS. However, staghorn blood vessels, myxoid or microcystic change, nuclear palisading, and interstitial mast cells are commonly observed in SFT. Malignant SFT can show dedifferentiation. However, unlike FS, lesional cells in SFT are positive for CD34 and STAT6 (18). MPNST can occur in the posterior mediastinum and shows fascicles of monotonous spindle cells with a “herringbone” pattern, mimicking FS (50). It also reveals alternating hypercellular and hypocellular zones with accentuated perivascular cellularity. Lesional cells are focally positive for S100 in 50% cases (53), and patchy positive for SOX10 (54). The majority of both sporadic (95%) and radiotherapy-related (91%) MPNSTs showed loss of H3K27me3 expression (55). Other mimickers such as cellular schwannoma, fibromatosis, LGFMS, and dedifferentiated liposarcoma might be misdiagnosed as adult FS (43). Morphological features of fibromatosis and LGFMS have been discussed above. Schwannoma most commonly occurs in the posterior mediastinum and presents as a well circumscribed mass. Lesional cells are diffusely positive for S100 and SOX10. Finally, low grade dedifferentiated liposarcoma may show spindle cell morphology which mimics FS. If well-differentiated liposarcoma is present in the background, the diagnosis of low grade dedifferentiated liposarcoma can generally be rendered without difficulty. For difficult cases, dedifferentiated liposarcoma typically shows expression of CDK4 and MDM2 and MDM2 amplification by FISH (34).
Conclusions
In summary, SFT and LGFMS show unique clinicopathological and molecular features that separate them from adult FS. Immunostaining and molecular testing are very helpful for rendering a definitive diagnosis of SFT and LGFMS. In contrast, adult FS is inherently a diagnosis of exclusion (43). A large number of mesenchymal and nonmesenchymal tumors may mimic adult FS, including MPNST, monophasic synovial sarcoma, SFT, spindle cell melanoma, sarcomatoid carcinoma, etc. Diagnosis of adult FS should be rendered only with the greatest of trepidation after all mimickers have been excluded through taking into account the clinical information, morphological features, immunostaining, molecular testing and possible electron microscopy study (43). So far there are no distinct immunohistochemical markers or molecular features that are pathognomonic for the diagnosis of adult FS and the underlying molecular genetics remain to be fully explored.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Saul Suster and David Suster) for the series “Mediastinal Sarcomas” published in Mediastinum. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-44). The series “Mediastinal Sarcomas” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Burt M, Ihde JK, Hajdu SI, et al. Primary sarcomas of the mediastinum: results of therapy. J Thorac Cardiovasc Surg 1998;115:671-80. [Crossref] [PubMed]
- Temes R, Allen N, Chavez T, et al. Primary mediastinal malignancies in children: report of 22 patients and comparison to 197 adults. Oncologist 2000;5:179-84. [Crossref] [PubMed]
- Temes R, Chavez T, Mapel D, et al. Primary mediastinal malignancies: findings in 219 patients. West J Med 1999;170:161-6. [PubMed]
- Cardillo G, Lococo F, Carleo F, et al. Solitary fibrous tumors of the pleura. Curr Opin Pulm Med 2012;18:339-46. [Crossref] [PubMed]
- van de Rijn M, Lombard CM, Rouse RV. Expression of CD34 by solitary fibrous tumors of the pleura, mediastinum, and lung. Am J Surg Pathol 1994;18:814-20. [Crossref] [PubMed]
- Witkin GB, Rosai J. Solitary fibrous tumor of the mediastinum. A report of 14 cases. Am J Surg Pathol 1989;13:547-57. [Crossref] [PubMed]
- Ronchi A, Cozzolino I, Zito Marino F, et al. Extrapleural solitary fibrous tumor: A distinct entity from pleural solitary fibrous tumor. An update on clinical, molecular and diagnostic features. Ann Diagn Pathol 2018;34:142-50. [Crossref] [PubMed]
- Weidner N. Solitary fibrous tumor of the mediastinum. Ultrastruct Pathol 1991;15:489-92. [Crossref] [PubMed]
- Bortolotti U, Calabro F, Loy M, et al. Giant intrapericardial solitary fibrous tumor. Ann Thorac Surg 1992;54:1219-20. [Crossref] [PubMed]
- Iwata T, Nishiyama N, Izumi N, et al. Solitary fibrous tumor of the thymus with local invasiveness and pleural dissemination: report of a case. Ann Thorac Cardiovasc Surg 2007;13:198-202. [PubMed]
- Gannon BR, O'Hara CD, Reid K, et al. Solitary fibrous tumor of the anterior mediastinum: a rare extrapleural neoplasm. Tumori 2007;93:508-10. [Crossref] [PubMed]
- Liu X, Zhang HY, Bu H, et al. Fat-forming variant of solitary fibrous tumor of the mediastinum. Chin Med J (Engl) 2007;120:1029-32. [Crossref] [PubMed]
- Zhao XG, Wang H, Wang YL, et al. Malignant solitary fibrous tumor of the right atrium. Am J Med Sci 2012;344:422-5. [Crossref] [PubMed]
- Xiang Y, Tu S, Zhang F. Rapid metastasis of mediastinal solitary fibrous tumor: Report a case. Medicine (Baltimore) 2017;96:e9307 [Crossref] [PubMed]
- Webb AJ, Yassin AS, Saeed A, et al. Mediastinal solitary fibrous tumor diagnosed by endobronchial ultrasound-directed biopsy. Am J Case Rep 2017;18:549-52. [Crossref] [PubMed]
- Zhang L, Liu X, Li X, et al. Diagnosis and surgical treatment of mediastinal solitary fibrous tumor. Asia Pac J Clin Oncol 2017;13:e473-80. [Crossref] [PubMed]
- Demicco EG, Park MS, Araujo DM, et al. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol 2012;25:1298-306. [Crossref] [PubMed]
- Doyle LA, Vivero M, Fletcher CD, et al. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol 2014;27:390-5. [Crossref] [PubMed]
- Cheah AL, Billings SD, Goldblum JR, et al. STAT6 rabbit monoclonal antibody is a robust diagnostic tool for the distinction of solitary fibrous tumour from its mimics. Pathology 2014;46:389-95. [Crossref] [PubMed]
- Saeed O, Zhang S, Cheng L, et al. STAT6 expression in solitary fibrous tumor and histologic mimics: a single institution experience. Appl Immunohistochem Mol Morphol 2020;28:311-5. [Crossref] [PubMed]
- Chmielecki J, Crago AM, Rosenberg M, et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet 2013;45:131-2. [Crossref] [PubMed]
- Mohajeri A, Tayebwa J, Collin A, et al. Comprehensive genetic analysis identifies a pathognomonic NAB2/STAT6 fusion gene, nonrandom secondary genomic imbalances, and a characteristic gene expression profile in solitary fibrous tumor. Genes Chromosomes Cancer 2013;52:873-86. [Crossref] [PubMed]
- Robinson DR, Wu YM, Kalyana-Sundaram S, et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet 2013;45:180-5. [Crossref] [PubMed]
- Yoshida A, Tsuta K, Ohno M, et al. STAT6 immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors. Am J Surg Pathol 2014;38:552-9. [Crossref] [PubMed]
- Gold JS, Antonescu CR, Hajdu C, et al. Clinicopathologic correlates of solitary fibrous tumors. Cancer 2002;94:1057-68. [Crossref] [PubMed]
- Reisenauer JS, Mneimneh W, Jenkins S, et al. Comparison of risk stratification models to predict recurrence and survival in pleuropulmonary solitary fibrous tumor. J Thorac Oncol 2018;13:1349-62. [Crossref] [PubMed]
- Espat NJ, Lewis JJ, Leung D, et al. Conventional hemangiopericytoma: modern analysis of outcome. Cancer 2002;95:1746-51. [Crossref] [PubMed]
- Mosquera JM, Fletcher CD. Expanding the spectrum of malignant progression in solitary fibrous tumors: a study of 8 cases with a discrete anaplastic component--is this dedifferentiated SFT? Am J Surg Pathol 2009;33:1314-21. [Crossref] [PubMed]
- Demicco EG, Wagner MJ, Maki RG, et al. Risk assessment in solitary fibrous tumors: validation and refinement of a risk stratification model. Mod Pathol 2017;30:1433-42. [Crossref] [PubMed]
- Evans HL. Low-grade fibromyxoid sarcoma. A report of two metastasizing neoplasms having a deceptively benign appearance. Am J Clin Pathol 1987;88:615-9. [Crossref] [PubMed]
- Evans HL. Low-grade fibromyxoid sarcoma. A report of 12 cases. Am J Surg Pathol 1993;17:595-600. [Crossref] [PubMed]
- Evans HL. Low-grade fibromyxoid sarcoma: a clinicopathologic study of 33 cases with long-term follow-up. Am J Surg Pathol 2011;35:1450-62. [Crossref] [PubMed]
- Folpe AL, Lane KL, Paull G, et al. Low-grade fibromyxoid sarcoma and hyalinizing spindle cell tumor with giant rosettes: a clinicopathologic study of 73 cases supporting their identity and assessing the impact of high-grade areas. Am J Surg Pathol 2000;24:1353-60. [Crossref] [PubMed]
- Mohamed M, Fisher C, Thway K. Low-grade fibromyxoid sarcoma: clinical, morphologic and genetic features. Ann Diagn Pathol 2017;28:60-7. [Crossref] [PubMed]
- Ud Din N, Ahmad Z, Zreik R, et al. Abdominopelvic and retroperitoneal low-grade fibromyxoid sarcoma: a clinicopathologic study of 13 cases. Am J Clin Pathol 2018;149:128-34. [Crossref] [PubMed]
- Galetta D, Cesario A, Margaritora S, et al. Primary mediastinal hyalinizing spindle cell tumor with giant rosettes. Ann Thorac Surg 2004;77:2206-9. [Crossref] [PubMed]
- Takanami I, Takeuchi K, Naruke M. Low-grade fibromyxoid sarcoma arising in the mediastinum. J Thorac Cardiovasc Surg 1999;118:970-1. [Crossref] [PubMed]
- Jakowski JD, Wakely PE Jr. Primary intrathoracic low-grade fibromyxoid sarcoma. Hum Pathol 2008;39:623-8. [Crossref] [PubMed]
- Maeda E, Ohta S, Watadani T, et al. Imaging findings of thoracic low-grade fibromyxoid sarcoma: report of three cases. Jpn J Radiol 2009;27:375-80. [Crossref] [PubMed]
- Doyle LA, Moller E, Dal Cin P, et al. MUC4 is a highly sensitive and specific marker for low-grade fibromyxoid sarcoma. Am J Surg Pathol 2011;35:733-41. [Crossref] [PubMed]
- Doyle LA, Wang WL, Dal Cin P, et al. MUC4 is a sensitive and extremely useful marker for sclerosing epithelioid fibrosarcoma: association with FUS gene rearrangement. Am J Surg Pathol 2012;36:1444-51. [Crossref] [PubMed]
- Fletcher C, Unni, KK, Mertens, F. WHO classification of tumours: pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press, 2002.
- Bahrami A, Folpe AL. Adult-type fibrosarcoma: a reevaluation of 163 putative cases diagnosed at a single institution over a 48-year period. Am J Surg Pathol 2010;34:1504-13. [Crossref] [PubMed]
- Hansen T, Katenkamp K, Brodhun M, et al. Low-grade fibrosarcoma--report on 39 not otherwise specified cases and comparison with defined low-grade fibrosarcoma types. Histopathology 2006;49:152-60. [Crossref] [PubMed]
- Folpe AL. Fibrosarcoma: a review and update. Histopathology 2014;64:12-25. [Crossref] [PubMed]
- March HW, Lovelock FJ, Brown H. Fibrosarcoma of the mediastinum. Dis Chest 1955;28:431-8. [Crossref] [PubMed]
- Barua NR, Patel AR, Takita H, et al. Fibrosarcoma of the mediastinum. J Surg Oncol 1979;12:11-7. [Crossref] [PubMed]
- Pescarmona E, Remotti D, Marzullo A, et al. Fibrosarcoma of the thymic region. A case report. Tumori 1991;77:363-6. [Crossref] [PubMed]
- Weiss SW. Proliferative fibroblastic lesions. From hyperplasia to neoplasia. Am J Surg Pathol 1986;10:14-25. [PubMed]
- Suster S, Moran CA. Primary synovial sarcomas of the mediastinum: a clinicopathologic, immunohistochemical, and ultrastructural study of 15 cases. Am J Surg Pathol 2005;29:569-78. [Crossref] [PubMed]
- Xu H, Koo HJ, Lim S, et al. Desmoid-type fibromatosis of the thorax: CT, MRI, and FDG PET characteristics in a large series from a tertiary referral center. Medicine (Baltimore) 2015;94:e1547 [Crossref] [PubMed]
- Marchevsky AM. Mediastinal tumors of peripheral nervous system origin. Semin Diagn Pathol 1999;16:65-78. [PubMed]
- Hirose T, Hasegawa T, Kudo E, et al. Malignant peripheral nerve sheath tumors: an immunohistochemical study in relation to ultrastructural features. Hum Pathol 1992;23:865-70. [Crossref] [PubMed]
- Kang Y, Pekmezci M, Folpe AL, et al. Diagnostic utility of SOX10 to distinguish malignant peripheral nerve sheath tumor from synovial sarcoma, including intraneural synovial sarcoma. Mod Pathol 2014;27:55-61. [Crossref] [PubMed]
- Prieto-Granada CN, Wiesner T, Messina JL, et al. Loss of H3K27me3 expression is a highly sensitive marker for sporadic and radiation-induced MPNST. Am J Surg Pathol 2016;40:479-89. [Crossref] [PubMed]
Cite this article as: Chen S, Badve SS. Fibroblastic sarcomas of the mediastinum. Mediastinum 2020;4:26.


