
Mediastinal sarcomas: experience using fine needle aspiration cytopathology
Introduction
Several options exist for diagnostic pathologic sampling of mediastinal neoplasms. Among these are the surgical approaches of thoracoscopy (including video-assisted thoracoscopic surgery, VATS), mediastinoscopy, and mediastinotomy, and the less invasive endobronchial ultrasound-guided (EBUS) biopsy, transesophageal endoscopic ultrasound-guided (EUS) biopsy, and percutaneous CT-guided biopsy (1,2). Location within the mediastinum (anterior, middle, posterior) often dictates which interventional approach is used. Based on literature citations, cytopathology, either fine needle aspiration (FNA) biopsy cytopathology, or imprint cytopathology from core needle specimens appears to be underutilized in the diagnosis of all forms of mediastinal neoplasia, but particularly so for sarcomas in large part due to their rarity. This is in marked contrast to the routine use of FNA cytology in mediastinal lymph node staging for lung cancer and lymphoma (3-5).
A 10-year SEER database review of primary mediastinal sarcomas found the category of “sarcoma, NOS” to be more common that any specific sarcoma subtype. According to the author, this reflects the difficulty in obtaining adequate tissue to determine a specific histopathological classification (6). Virtually every sarcoma has been described in the mediastinum, but such instances are rare and typically the subject of small series and case reports. Thus, the cytopathology literature regarding these sarcomas would be expected to be scant also. To wit, one of the largest studies of mediastinal FNA biopsy consisting of 189 cases (71% of which represented neoplasms) from three separate institutions featured no examples of sarcoma (7). Several other studies reflecting the paucity of cytologic evaluation of mediastinal sarcomas include one with 102 mediastinal neoplasms only 2.9% of which were sarcomas (8), and another with 42 mediastinal aspirates but only 2 sarcomas (9). Marcus et al. reported that 4% of their 107 mediastinal fine needle aspirates were soft tissue tumors, but failed to specify whether they represented sarcomas or benign soft tissue neoplasms (10). Incomplete sampling remains a critical source of error in any type of biopsy procedure. Since various sarcomas may arise as components of mediastinal germ cell tumors, one must be cognizant of this possibility also (11). The purpose of this review is to spotlight the cytopathology of select examples of mediastinal sarcomas.
Diagnostic categories
Liposarcoma (LPS)
LPS is regarded as the most common mesenchymal sarcoma of the mediastinum (6,12,13). It occurs in all three mediastinal compartments, but primarily the anterior and posterior mediastinum. Each of the major subtypes of LPS (well-differentiated, myxoid, dedifferentiated, and pleomorphic) has been reported in this location, but well-differentiated and de-differentiated subtypes appear to be most common (13). The cytopathology mimics that seen in the somatic soft tissues. Two reports of primary mediastinal myxoid LPS and one of metastasis to the mediastinum showed similar features consisting of myxoid stroma, cytologically monotonous bland nuclei, variable cytoplasmic vacuolization, and arborizing thin capillaries (14-16) (Figure 1). Another example of primary mediastinal LPS described both myxoid and pleomorphic features, but failed to illustrate any cytologic images (17). Rather than being multi-vacuolated, the lipoblasts in myxoid LPS usually contain a single vacuole (18). A study of 39 LPS by Kapila et al. concluded that the role of FNA in identification of specific variants of LPS is limited (19). In more recent series, the added use of confirmatory FISH analysis for DDIT3 gene rearrangement is not only possible from smears and cytology cell-blocks, but also valuable for correctly confirming this diagnosis and distinguishing it from other myxoid neoplasms (20).
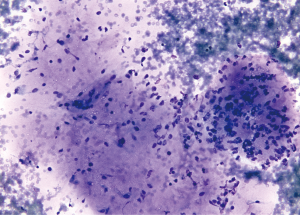
Pleomorphic LPS (PLPS) is the least common form of LPS representing up to 5% of all LPS (21), and descriptions of its cytopathology are extremely rare in the mediastinum (22). Cytologic smears of PLPS are those of a high-grade sarcoma, but have been mistaken rarely by experienced observers as metastatic carcinoma (23). Aspirates are typically hypercellular containing isolated malignant cells and cell aggregates with striking anisonucleosis, spindle cells, epithelioid cells, multinucleated tumor giant cells, coarse chromatin, and cytoplasmic lipid-filled vacuoles (22,24) (Figure 2). A single mediastinal case reporting the cytopathology described fusiform cells with nuclear pleomorphism and cytoplasmic macrovacuoles (25). Unfortunately, this cytomorphology is less than specific overlapping to a considerable degree with other pleomorphic sarcoma subtypes [e.g., undifferentiated pleomorphic sarcoma, dedifferentiated LPS (DDLPS)]. As PLPS has no specific molecular signature or immunohistochemical (IHC) marker, distinction among these pleomorphic sarcomas is extremely challenging in cytology aspirates. The identification of large multivacuolated lipoblasts is critical to diagnostic recognition of PLPS; unfortunately, these cells can vary from <1% to 80% of the tumor area (21). Mariño-Enríquez et al. found variable and overlapping cytologic features in their large study comparing DDLPS and PLPS (including 1 mediastinal primary and 1 mediastinal metastasis) (22). They concluded that only coexpression of mdm2 and cdk4 IHC in the former helped to distinguish between the two tumors.
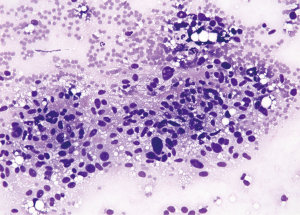
An analogous problem exists in distinguishing well-differentiated LPS in aspirate smears from those of lipoma since the obligatory hyperchromic atypical cells necessary for diagnosis of the former may be widely scattered in an otherwise non-specific lipomatous background (23,26-28). A series using combined FNA and core needle biopsy on 67 non-mediastinal LPS cases showed a diagnostic yield using FNA alone in accurately determining subtype for all forms of LPS to be 64%. However, that yield dropped to 39% for recognition of well-differentiated LPS compared to 94% for myxoid LPS (29). Einarsdóttir et al. stressed the importance of comparison with image findings. Those tumors with a fat content <75% of tumor volume were more apt to represent LPS, and a diagnoses of lipoma or atypical lipoma should be questioned (26). Even more problematic is the example of mistaking mediastinal thymolipoma for well-differentiated LPS (30), or the possibility of misidentifying the multinucleated floret-type cells of pleomorphic lipoma for LPS (23,31).
Vascular sarcomas
Although many studies have described the cytomorphology of angiosarcoma (AS) and epithelioid hemangioendothelioma (EHE), no set of features is unequivocally diagnostic. Mediastinal EHE is rare (32,33). A large FNA series of EHE (14 cases) contained two examples in the mediastinum, one primary and one metastasis to a mediastinal lymph node (34). Both examples were diagnosed incorrectly cytologically as metastatic carcinoma. The series of VandenBussche et al. (15 cases) also contained two aspirates from the mediastinum (35). Cytomorphology consists of polygonal/epithelioid and even spindle-shaped cells dispersed in hypercellular clusters and as single forms. Nuclei are rounded or oval, hyperchromatic, eccentrically located in the cell resembling plasmacytoid or signet ring cells, and possess intranuclear cytoplasmic pseudoinclusions which may be common (34-36) (Figure 3). Cell cytoplasm is moderate in amount with single or multiple vacuoles. Only rarely are lumina containing entrapped intact and degenerating red cells present (34-36). In the mediastinum, the morphologic combination of cells in loose or tight clusters, an epithelioid configuration, and easily visible cytoplasmic vacuoles provides a strong mimic for adenocarcinoma or mesothelioma—tumors with which EHE is commonly confused (37-40). Most cytology reports fail to highlight the myxohyaline stroma that is commonly emphasized in histopathologic descriptions of EHE. Matrix material was found in only 20% of cases from the largest series (35). Definitive cytologic diagnosis of EHE requires awareness of the entity (often overlooked due to its rarity), and the application of IHC markers (CD31, ERG, CAMTA1) and/or FISH testing for WWTR1-CAMTA1 rearrangement (present in >90% of cases) from a cytology cell-block.
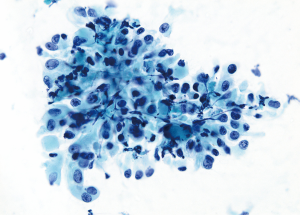
In a study comparing the cytopathology of EHE with that of epithelioid angiosarcoma (EAS), VandenBussche et al. concluded that many cytomorphological features existed on a spectrum that overlapped considerably between the two (35). While nuclear pleomorphism varied among both tumors, only EAS cases exhibited marked nuclear pleomorphism, and about half of EHE cases contained nuclear grooves which were lacking in all EAS examples (35). Some authors describe rhabdoid morphology to the cells in EAS (41,42). Mitoses, typically readily found in EAS tissue specimens, cannot be relied upon in FNA specimens. They were absent or rare in both EHE and EAS cases from one study (35); however, another found abnormal mitoses in 85% of cases (43). Although the WHO classification of soft tissue tumors does not recognize specific subtypes of AS, the more common histo-and cyto-pathology of AS is that of a heterogenous cell population with a high percentage of spindle cells (44) along with epithelioid and giant cells in variable numbers (41,43,45). In some cases, smears may be hypocellular due to the dilutional effect of abundant blood being aspirated. The anastomosing vascular channels seen in tissue are, of course, usually invisible in cytologic preparations. Most aspirates display 3-dimensional clusters with moderate-marked nucleomegaly and nuclear pleomorphism (41,43) (Figure 4). Presumptive vasoformative features consisting of microacinar structures, arborizing microtissue fragments, intracytoplasmic lumina, signet-ring-like cells, and rare erythrophagocytosis have been emphasized by some authors (43-45). It is important to remember that both EHE and AS may stain with pan-keratin markers and EMA (particularly strong staining in EAS) in addition to previously alluded endothelial markers so a complete IHC panel is required (46).
Malignant peripheral nerve sheath tumor (MPNST)
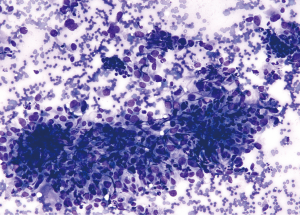
MPNST is a rare mediastinal malignancy, and when present primarily affects the posterior mediastinum. The three largest FNA cytology series of MPNST (82 total cases) mention only a single case from the mediastinum, although several cases are described as involving the chest wall (47-49).
Cytologic smears are moderately to highly cellular, but occasionally may be hypocellular. Spindle cells are dispersed both as individual forms, and in thinly or densely concentrated aggregates. Parallel orientation may commonly produce a fascicular pattern in smears. Nuclei are fusiform or blunt-ended with a smooth contour (Figure 5). Some authors have underscored that comma-shaped cells with twisted or wavy nuclei are the most reliable morphologic feature for suspecting MPNST (48); however, others note that “wavy” or “kinked” nuclear contour is an uncommon feature (47,50). There is usually some degree of anisonucleosis, but marked pleomorphism is seen only in high-grade/anaplastic MPNST. Cytoplasm is variable, usually sparse and tapered. Smear background is often “clean”, but individual cell necrosis may be present.
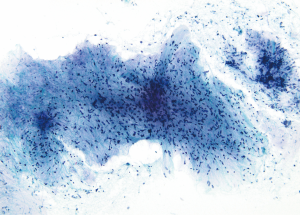
Primary conventional MPNST is extremely difficult to differentiate from other spindle cell malignancies without either a clinical history of NF-1, or knowledge that the neoplasm is in proximity to or appears to arise from a major nerve. To illustrate, none of eight (50), 1 of 13 (8%) (48), none of three (49), and 8 of 27 (30%) cases (47) of primary MPNST were correctly recognized using FNA cytology. Conversely, the ability to recognize MPNST in recurrent tumors is very high with a correct diagnosis in 93% of recurrent cases in the largest study (47). Smears of low-intermediate grade MPNST may be mistaken for other spindle cell sarcomas, and even schwannoma, particularly when “ancient” change with nuclear atypia is present (51). High-grade MPNST merely demonstrates the non-specific cytopathology of a pleomorphic sarcoma. The epithelioid variant of MPNST imitates a variety of mesenchymal and non-mesenchymal epithelioid neoplasms including malignant melanoma, epithelioid sarcoma, chordoma, and metastatic carcinoma (52). Ancillary IHC staining of conventional MPNST shows staining of S-100 and SOX-10 in only about half of cases, but since this staining is typically patchy it may be missed in a cytology cell-block specimen. Complete loss of staining with H3K27me3 antibody may assist with the diagnosis. However, this loss occurs more often in high-grade MPNST than in low-grade cases (53). In contrast, epithelioid MPNST exhibits strong and diffuse S100 and SOX1 staining, but without expression of SMARCB-1 and melanoma markers (54).
Unclassified sarcomas/ sarcomas of uncertain differentiation
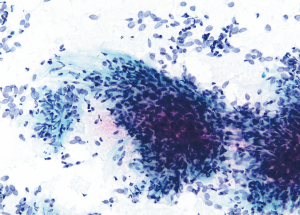
Mediastinal synovial sarcoma (SS) is rare accounting for about 9% of intrathoracic SSs in one series (55). Most tumors occur in patients <50 years of age with monophasic SS being far more common than biphasic SS (56). Only single case reports constitute the FNA cytology of mediastinal SS (57,58). Large series of SS from soft tissue sites show hypercellular smears with cell clusters so thick that one cannot appreciate individual cells within these 3-dimensional aggregates. Relatively uniform rounded to spindle shaped cells have oval to oblong monotonous nuclei. These have evenly dispersed chromatin, inconspicuous to absent nucleoli, and smooth contours combined with scant non-vacuolated cytoplasm (Figure 6). Stripped nuclei are common and unless one captures a necrotic focus, there is minimal background stroma (59-62). The biphasic variant is difficult to appreciate on smears since these glandular structures which remain intact in tissue sections are often disrupted using FNA. A specific cytologic diagnosis is possible in >95% of primary neoplasms when ancillary IHC staining incorporating TLE-1 as part of a panel of stains is paired with fluorescence in-situ hybridization (FISH) or polymerase chain reaction (PCR) testing for SYT gene rearrangement (62).
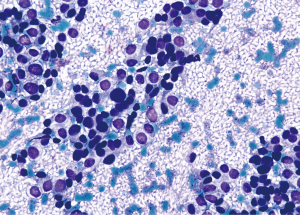
Rare examples of “round cell” sarcomas have been reported as primary mediastinal neoplasms. The most common among these is the Ewing sarcoma/family of tumors (ES). As a mediastinal primary, ES is exceptionally rare in contrast to its more common thoracopulmonary location (63,64). Aspiration cytopathology consists of highly cellular smears with monotonous cells about 2–3 times the diameter of a mature lymphocyte dispersed individually with a minority in loose clusters. Nuclear molding and rarely acinar or pseudorosette formation may be seen. Nuclei are rounded to oval with indistinct nucleoli. Cell cytoplasm is usually meager, but not infrequently contains glycogenated cytoplasmic vacuoles. If numerous, these create a so-called “tigroid” background with naked strips of cytoplasm arranged in parallel or as a plexiform network (28,65,66) (Figure 7). Due to morphologic mimicry with other malignant “small” rounded cell sarcomas, genetic confirmation is nearly always required for a definitive diagnosis. This is often accomplished with FISH testing from a cytology cell-block. Most ES cases have the EWSR1-FLI1 fusion, about 10% harbor fusion of the EWSR1-ERG genes, and a smaller percentage partner with other ETS-family fusion genes. In addition to the non-specific, but still helpful CD99 staining, the newer NKX2.2 IHC stain has added greater specificity to recognizing ES (67). A rare cytologic example of CIC-rearranged sarcoma, CIC-DUX4, has been described in the mediastinum (68). The few reported examples of this sarcoma state that the cytopathology is somewhat more atypical than classic ES (68,69).
Conclusions
Based on citations from the English-based literature FNA cytology is uncommonly employed as a diagnostic modality for evaluating mediastinal sarcomas. Nonetheless, when cell morphology is paired with ancillary IHC staining and molecular methods (specifically FISH testing in selected sarcomas) diagnostic accuracy can be quite high and of clinical value.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Saul Suster and David Suster) for the series “Mediastinal Sarcomas” published in Mediastinum. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-30). The series “Mediastinal Sarcomas” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dvorak P, Hoffmann P, Kocova E, et al. CT-guided biopsy of the mediastinal masses. Can anatomical relationships predict complications? Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2019;163:220-6. [Crossref] [PubMed]
- Vilmann P, Krasnik M, Larsen SS, et al. Transesophageal endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) biopsy: a combined approach in the evaluation of mediastinal lesions. Endoscopy 2005;37:833-9. [Crossref] [PubMed]
- Karunamurthy A, Cai G, Dacic S, et al. Evaluation of endobronchial ultrasound-guided fine-needle aspirations (EBUS-FNA): correlation with adequacy and histologic follow-up. Cancer Cytopathol 2014;122:23-32. [Crossref] [PubMed]
- Labarca G, Sierra-Ruiz M, Kheir F, et al. Diagnostic accuracy of endobronchial ultrasound transbronchial needle aspiration in lymphoma. A systematic review and meta-analysis. Ann Am Thorac Soc 2019;16:1432-9. [Crossref] [PubMed]
- Tyan CC, Machuca T, Czarnecka K, et al. Performance of endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of isolated mediastinal and hilar lymphadenopathy. Respiration 2017;94:457-64. [Crossref] [PubMed]
- Abdel-Rahman O. An analysis of clinical characteristics and patient outcomes in primary mediastinal sarcomas. Expert Rev Anticancer Ther 2017;17:1071-6. [Crossref] [PubMed]
- Powers CN, Silverman JF, Geisinger KR, et al. Fine-needle aspiration biopsy of the mediastinum. A multi-institutional analysis. Am J Clin Pathol 1996;105:168-73. [Crossref] [PubMed]
- Goel D, Prayaga AK, Sundaram C, et al. Utility of fine needle aspiration cytology in mediastinal lesions: a clinicopathologic study of 1617 cases from a single institution. Acta Cytol 2008;52:404-11. [Crossref] [PubMed]
- Shabb NS, Fahl M, Shabb B, et al. Fine-needle aspiration of the mediastinum: a clinical, radiologic, cytologic, and histologic study of 42 cases. Diagn Cytopathol 1998;19:428-36. [Crossref] [PubMed]
- Marcus A, Narula N, Kamel MK, et al. Sensitivity and specificity of fine needle aspiration for the diagnosis of mediastinal lesions. Ann Diagn Pathol 2019;39:69-73. [Crossref] [PubMed]
- Malagon HD, Valdez AM, Moran CA, et al. Germ cell tumors with sarcomatous components: a clinicopathologic and immunohistochemical study of 46 cases. Am J Surg Pathol 2007;31:1356-62. [Crossref] [PubMed]
- Coindre JM. Liposarcoma. In: Travis WD, Brambilla E, Burke AP, et al. editors. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th edition. Lyon: IARC Press, 2015:290-1.
- Hahn HP, Fletcher CD. Primary mediastinal liposarcoma: clinicopathologic analysis of 24 cases. Am J Surg Pathol 2007;31:1868-74. [Crossref] [PubMed]
- Attal H, Jensen J, Reyes CV. Myxoid liposarcoma of the anterior mediastinum. Diagnosis by fine needle aspiration biopsy. Acta Cytol 1995;39:511-3. [PubMed]
- Munjal K, Pancholi V, Rege J, et al. Fine needle aspiration cytology in mediastinal myxoid liposarcoma: a case report. Acta Cytol 2007;51:456-8. [Crossref] [PubMed]
- Inuganti RV, Bala SG, Bharathi KY. Metastatic myxoid liposarcoma of lung and mediastinum diagnosed by fine needle aspiration. J Cytol 2011;28:33-5. [Crossref] [PubMed]
- Greif J, Marmor S, Merimsky O, et al. Primary liposarcoma of the mediastinum. Sarcoma 1998;2:205-7. [Crossref] [PubMed]
- Kilpatrick SE, Ward WG, Bos GD. The value of fine-needle aspiration biopsy in the differential diagnosis of adult myxoid sarcoma. Cancer 2000;90:167-77. [Crossref] [PubMed]
- Kapila K, Ghosal N, Gill SS, et al. Cytomorphology of lipomatous tumors of soft tissue. Acta Cytol 2003;47:555-62. [Crossref] [PubMed]
- Wakely PE Jr, Jin M. Myxoid liposarcoma: Fine-needle aspiration cytopathology in the molecular era. A report of 24 cases. J Am Soc Cytopathol 2016;5:162-9. [Crossref] [PubMed]
- Hornick JL, Bosenberg MW, Mentzel T, et al. Pleomorphic liposarcoma: clinicopathologic analysis of 57 cases. Am J Surg Pathol 2004;28:1257-67. [Crossref] [PubMed]
- Mariño-Enríquez A, Hornick JL, Dal Cin P, et al. Dedifferentiated liposarcoma and pleomorphic liposarcoma: a comparative study of cytomorphology and MDM2/CDK4 expression on fine-needle aspiration. Cancer Cytopathol 2014;122:128-37. [Crossref] [PubMed]
- Akerman M, Rydholm A. Aspiration cytology of lipomatous tumors: a 10-year experience at an orthopedic oncology center. Diagn Cytopathol 1987;3:295-302. [Crossref] [PubMed]
- Dodd LG, Jiang X, Rao K, et al. Pleomorphic liposarcoma: a cytologic study of five cases. Diagn Cytopathol 2015;43:138-43. [Crossref] [PubMed]
- Romero-Guadarrama MB, Jimenez-Becerra S, Duran-Padilla MA, et al. Mediastinal pleomorphic liposarcoma diagnosed by fine needle aspiration biopsy: a case report. Acta Cytol 2007;51:440-2. [Crossref] [PubMed]
- Einarsdóttir H, Skoog L, Söderlund V, et al. Accuracy of cytology for diagnosis of lipomatous tumors: comparison with magnetic resonance and computed tomography findings in 175 cases. Acta Radiol 2004;45:840-6. [Crossref] [PubMed]
- Klijanienko J, Caillaud JM, Lagacé R. Fine-needle aspiration in liposarcoma: cytohistologic correlative study including well-differentiated, myxoid, and pleomorphic variants. Diagn Cytopathol 2004;30:307-12. [Crossref] [PubMed]
- Kilpatrick SE, Geisinger KR. Soft tissue sarcomas: the usefulness and limitations of fine-needle aspiration biopsy. Am J Clin Pathol 1998;110:50-68. [Crossref] [PubMed]
- Nikolaidis P, Silverman SG, Cibas ES, et al. Liposarcoma subtypes: identification with computed tomography and ultrasound-guided percutaneous needle biopsy. Eur Radiol 2005;15:383-9. [Crossref] [PubMed]
- Romero-Guadarrama MB, Durán-Padilla MA, Cruz-Ortíz H, et al. Diagnosis of thymolipoma with fine needle aspiration biopsy. Report of a case initially misdiagnosed as liposarcoma. Acta Cytol 2004;48:441-6. [Crossref] [PubMed]
- Mao YQ, Liu XY, Han Y. Pleomorphic lipoma in the anterior mediastinum: A case report. World J Clin Cases 2019;7:2899-904. [Crossref] [PubMed]
- Suster S, Moran CA, Koss MN. Epithelioid hemangioendothelioma of the anterior mediastinum. Clinicopathologic, immunohistochemical, and ultrastructural analysis of 12 cases. Am J Surg Pathol 1994;18:871-81. [Crossref] [PubMed]
- Sardaro A, Bardoscia L, Petruzzelli MF, et al. Epithelioid hemangioendothelioma: an overview and update on a rare vascular tumor. Oncol Rev 2014;8:259. [PubMed]
- Chen Y, Chen JQ, Katz RL. Epithelioid hemangioendothelioma: a study of 14 cytopathology cases. J Am Soc Cytopathol 2015;4:148-59. [Crossref] [PubMed]
- VandenBussche CJ, Wakely PE Jr, Siddiqui MT, et al. Cytopathologic characteristics of epithelioid vascular malignancies. Acta Cytol 2014;58:356-66. [Crossref] [PubMed]
- Murali R, Zarka MA, Ocal IT, et al. Cytologic features of epithelioid hemangioendothelioma. Am J Clin Pathol 2011;136:739-46. [Crossref] [PubMed]
- Nowels KW, Burford-Foggs A, Benson AB 3rd, et al. Epithelioid hemangioendothelioma: cytomorphology and histological features of a case. Diagn Cytopathol 1989;5:75-8. [Crossref] [PubMed]
- Buggage RR, Soudi N, Olson JL, et al. Epithelioid hemangioendothelioma of the lung: pleural effusion cytology, ultrastructure, and brief literature review. Diagn Cytopathol 1995;13:54-60. [Crossref] [PubMed]
- Antic T, Staerkel G. Mediastinal epithelioid hemangioendothelioma metastatic to lymph nodes and pleural fluid: report of a case. Diagn Cytopathol 2010;38:113-6. [PubMed]
- Ryu HS, Lee SS, Choi HS, et al. A case of pulmonary malignant epithelioid hemangioendothelioma misdiagnosed as adenocarcinoma by fine needle aspiration cytology. Diagn Cytopathol 2011;39:801-7. [Crossref] [PubMed]
- Klijanienko J, Caillaud JM, Lagacé R, et al. Cytohistologic correlations in angiosarcoma including classic and epithelioid variants: Institut Curie's experience. Diagn Cytopathol 2003;29:140-5. [Crossref] [PubMed]
- Wakely PE Jr, Frable WJ, Kneisl JS. Aspiration cytopathology of epithelioid angiosarcoma. Cancer 2000;90:245-51. [Crossref] [PubMed]
- Geller RL, Hookim K, Sullivan HC, et al. Cytologic features of angiosarcoma: A review of 26 cases diagnosed on FNA. Cancer Cytopathol 2016;124:659-68. [Crossref] [PubMed]
- Minimo C, Zakowski M, Lin O. Cytologic findings of malignant vascular neoplasms: a study of twenty-four cases. Diagn Cytopathol 2002;26:349-55. [Crossref] [PubMed]
- Boucher LD, Swanson PE, Stanley MW, et al. Cytology of angiosarcoma. Findings in fourteen fine-needle aspiration biopsy specimens and one pleural fluid specimen. Am J Clin Pathol 2000;114:210-9. [Crossref] [PubMed]
- Sullivan HC, Edgar MA, Cohen C, et al. The utility of ERG, CD31 and CD34 in the cytological diagnosis of angiosarcoma: an analysis of 25 cases. J Clin Pathol 2015;68:44-50. [Crossref] [PubMed]
- Wakely PE Jr, Ali SZ, Bishop JA. The cytopathology of malignant peripheral nerve sheath tumor: a report of 55 fine-needle aspiration cases. Cancer Cytopathol 2012;120:334-41. [Crossref] [PubMed]
- Klijanienko J, Caillaud JM, Lagacé R, et al. Cytohistologic correlations of 24 malignant peripheral nerve sheath tumor (MPNST) in 17 patients: the Institut Curie experience. Diagn Cytopathol 2002;27:103-8. [Crossref] [PubMed]
- Jiménez-Heffernan JA, López-Ferrer P, Vicandi B, et al. Cytologic features of malignant peripheral nerve sheath tumor. Acta Cytol 1999;43:175-83. [Crossref] [PubMed]
- Gupta K, Dey P, Vashisht R. Fine-needle aspiration cytology of malignant peripheral nerve sheath tumors. Diagn Cytopathol 2004;31:1-4. [Crossref] [PubMed]
- Henke AC, Salomao DR, Hughes JH. Cellular schwannoma mimics a sarcoma: an example of a potential pitfall in aspiration cytodiagnosis. Diagn Cytopathol 1999;20:312-6. [Crossref] [PubMed]
- Jiwani S, Gokden M, Lindberg M, et al. Fine-needle aspiration cytology of epithelioid malignant peripheral nerve sheath tumor: A case report and review of the literature. Diagn Cytopathol 2016;44:226-31. [Crossref] [PubMed]
- Lu VM, Marek T, Gilder HE, et al. H3K27 trimethylation loss in malignant peripheral nerve sheath tumor: a systematic review and meta-analysis with diagnostic implications. J Neurooncol 2019;144:433-43. [Crossref] [PubMed]
- Rekhi B, Kosemehmetoglu K, Tezel GG, et al. Clinicopathologic features and immunohistochemical spectrum of 11 cases of epithelioid malignant peripheral nerve sheath tumors, including INI1/SMARCB1 results and BRAF V600E analysis. APMIS 2017;125:679-89. [Crossref] [PubMed]
- Hartel PH, Fanburg-Smith JC, Frazier AA, et al. Primary pulmonary and mediastinal synovial sarcoma: a clinicopathologic study of 60 cases and comparison with five prior series. Mod Pathol 2007;20:760-9. [Crossref] [PubMed]
- Terra S, Aesif SW, Maleszewski JJ, et al. Mediastinal synovial sarcoma: Clinicopathologic analysis of 21 cases with molecular confirmation. Am J Surg Pathol 2018;42:761-6. [Crossref] [PubMed]
- Huang CC, Michael CW, Pang JC. Fine needle aspiration of primary mediastinal synovial sarcoma: cytomorphologic, immunohistochemical, and molecular study. Diagn Cytopathol 2014;42:170-6. [Crossref] [PubMed]
- Yoshioka S, Ebisu Y, Ishida M, et al. Fine-needle aspiration cytology of primary mediastinal synovial sarcoma: A case report with an immunocytochemical approach. Diagn Cytopathol 2020;48:499-501. [Crossref] [PubMed]
- Kilpatrick SE, Teot LA, Stanley MW, et al. Fine-needle aspiration biopsy of synovial sarcoma. A cytomorphologic analysis of primary, recurrent, and metastatic tumors. Am J Clin Pathol 1996;106:769-75. [Crossref] [PubMed]
- Klijanienko J, Caillaud JM, Lagacé R, et al. Cytohistologic correlations in 56 synovial sarcomas in 36 patients: the Institut Curie experience. Diagn Cytopathol 2002;27:96-102. [Crossref] [PubMed]
- Ryan MR, Stastny JF, Wakely PE Jr. The cytopathology of synovial sarcoma: a study of six cases, with emphasis on architecture and histopathologic correlation. Cancer 1998;84:42-9. [Crossref] [PubMed]
- Zhang Y, Wessman S, Wejde J, et al. Diagnosing synovial sarcoma by fine-needle aspiration cytology and molecular techniques. Cytopathology 2019;30:504-9. [Crossref] [PubMed]
- Javery O, Krajewski K, O'Regan K, et al. A to Z of extraskeletal Ewing sarcoma family of tumors in adults: imaging features of primary disease, metastatic patterns, and treatment responses. AJR Am J Roentgenol 2011;197:W1015-22 [Crossref] [PubMed]
- Bae SH, Hwang JH, Da Nam B, et al. Multiple Ewing Sarcoma/Primitive Neuroectodermal Tumors in the Mediastinum: A Case Report and Literature Review. Medicine 2016;95:e2725 [Crossref] [PubMed]
- Klijanienko J, Couturier J, Bourdeaut F, et al. Fine-needle aspiration as a diagnostic technique in 50 cases of primary Ewing sarcoma/peripheral neuroectodermal tumor. Institut Curie's experience. Diagn Cytopathol 2012;40:19-25. [Crossref] [PubMed]
- Guiter GE, Gamboni MM, Zakowski MF. The cytology of extraskeletal Ewing sarcoma. Cancer 1999;87:141-8. [Crossref] [PubMed]
- Machado I, Yoshida A, Morales MGN, et al. Review with novel markers facilitates precise categorization of 41 cases of diagnostically challenging, "undifferentiated small round cell tumors". A clinicopathologic, immunophenotypic and molecular analysis. Ann Diagn Pathol 2018;34:1-12. [Crossref] [PubMed]
- Chebib I, Jo VY. Round cell sarcoma with CIC-DUX4 gene fusion: Discussion of the distinctive cytomorphologic, immunohistochemical, and molecular features in the differential diagnosis of round cell tumors. Cancer Cytopathol 2016;124:350-61. [Crossref] [PubMed]
- Tang S, Dodd LG. CIC-DUX4 sarcoma diagnosed by fine-needle aspiration cytology: A case report. Diagn Cytopathol 2018;46:958-63. [Crossref] [PubMed]
Cite this article as: Lott-Limbach AA, Wakely PE Jr. Mediastinal sarcomas: experience using fine needle aspiration cytopathology. Mediastinum 2020;4:14.


