
Primary mediastinal synovial sarcomas
Introduction
Synovial sarcoma derives its name from the initial assumption that the tumor originated from synovial cells, after a frequent association with joints, tendons and bursal structures was noted in early reports (1,2). Recognition of cases not associated with such structures and identification of variable epithelial differentiation in these tumors has subsequently led investigators to believe that synovial sarcomas are likely derived from pluripotent mesenchymal cells capable of divergent differentiation (3,4). Synovial sarcomas most commonly arise from the deep soft tissues of the extremities, accounting for 5–10% of all soft tissue sarcomas (5,6). These tumors primarily affect a younger patient population, including adolescents and young adults (7,8). Apart from the soft tissues, synovial sarcomas have been recognized in most anatomic sites, including the thoracic cavity. Most of the thoracic cases occur in the pleuropulmonary system while the mediastinum is an exceptionally rare site (9-13). In largest series to date, reporting 21 cases, the incidence of mediastinal synovial sarcomas was estimated at 11.2% of all thoracic synovial sarcomas (11). Histologically, synovial sarcomas can display a spectrum of growth patterns, including monophasic, biphasic and poorly differentiated types, adding diagnostic difficulty to their recognition, especially in sites not commonly associated with these neoplasms. The discovery that 95% of synovial sarcomas are characterized by a specific translocation t(X;18)(p11;q11) that can be detected by fluorescence in situ hybridization (FISH) or reverse transcription polymerase chain reaction (RT-PCR) is a powerful tool in the differential diagnosis with other, more common neoplasms in the mediastinum and molecular confirmation remains the gold standard in the diagnosis of these tumors. This review provides a summary of the current knowledge concerning these unusual tumors and compares their clinicopathologic characteristics to those of their soft tissue analogues.
Clinical features
Synovial sarcomas of the mediastinum can occur in any age group (range, 3–83 years) but are most commonly detected in the 4th decade of life. Contrary to their soft tissue analogues which show no gender bias, mediastinal primaries are more common among male patients (9-13). Although the spectrum of presenting symptoms is wide (Table 1), most patients complain of chest or shoulder pain, shortness of breath, cough and pericardial effusion. Notably, each patient’s clinical history and physical examination should include assessment of prior history or concurrent soft tissue tumor in order to exclude a metastatic process.
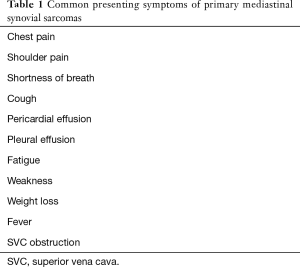
Full table
Radiological features
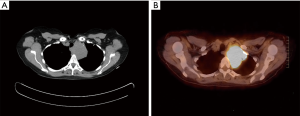
Mediastinal synovial sarcomas can arise in any mediastinal compartment, most commonly the anterior mediastinum, followed by the posterior mediastinum and middle and superior compartments (10,11,13). The radiological appearance of mediastinal synovial sarcomas is non-specific, often precluding separation from other mediastinal neoplasms. On chest radiographs, the tumors appear as well circumscribed neoplasms with sharply marginated borders or as ill-defined infiltrative lesions. Computed tomography (CT) scanning will reveal large tumor masses with homogeneous or heterogeneous enhancement that show high uptake on positron emission tomography (PET) (Figure 1A,B). At magnetic resonance imaging (MRI), the tumors typically demonstrate heterogeneous signal intensity on T1- and T2-weighted images and may contain fluid-fluid levels due to hemorrhage or necrosis within cystic tumor components. Cyst formation, areas of calcification, necrosis and hemorrhage are common findings (10,12,14).
Pathological features
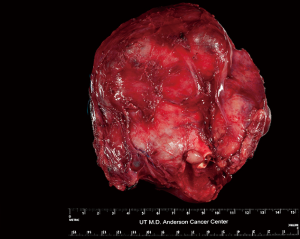
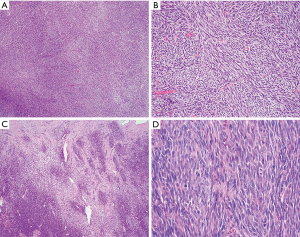
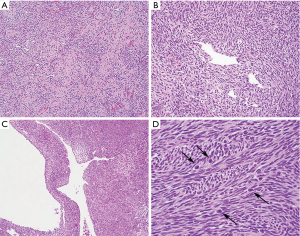
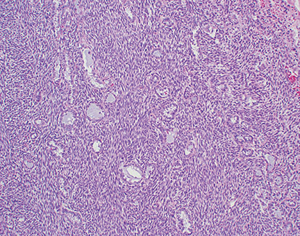
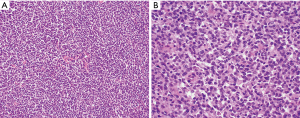
Grossly, synovial sarcomas arising in the mediastinum are large tumors with reported sizes ranging from 5 to 23 cm and an average size around 12 cm (9-11). The tumors may be well circumscribed and surrounded by a thin fibrous capsule or poorly delineated masses that infiltrate surrounding anatomic structures (Figure 2). The cut surface is gray-white to tan and can have a soft or firm consistency. Areas of calcification, cystic degeneration, gelatinous change, hemorrhage or necrosis can often be identified in varying proportions (9,10). Histologically, the tumors can be divided into three different subtypes: monophasic, biphasic and poorly differentiated synovial sarcoma. Monophasic tumors are characterized by a monotonous, highly cellular spindle cell proliferation growing in sheets, fascicles or storiform patterns (Figure 3A,B). In some areas, a palisading nuclear pattern reminiscent of Verocay bodies or a tigroid appearance can be observed and alternating hypo and hypercellular fascicles are also very common (Figure 3C). Individual tumor cells have fusiform nuclei with indistinct nucleoli and a scant rim of eosinophilic cytoplasm. Cytologic atypia is not a feature of these tumors but the mitotic activity is typically increased, ranging from 2 to more than 20 mitoses per 10 high power fields (HPF) (Figure 3D). Frequent stromal changes include myxoid change or hyalinization (Figure 4A), a hemangiopericytic (staghorn) vascular pattern (Figure 4B), foci of calcification, cystic change (Figure 4C), and areas of necrosis or hemorrhage. Another characteristic finding is the presence of scattered mast cells among the tumor cells (9-13) (Figure 4D). The biphasic variant contains an additional epithelioid component that can comprise 20–80% of the tumor volume (9). These epithelioid elements consist of nests, clefts, papillary formations and glandular structures and are composed of round, cuboidal or columnar cells with round to oval nuclei, inconspicuous nucleoli and abundant eosinophilic cytoplasm (Figure 5). In cases with glandular structures, the lumina may be filled with homogeneous eosinophilic material (9-13). Poorly differentiated tumors can manifest as proliferations of small round blue cells, large epithelioid/rhabdoid cells or high-grade spindle cells (11,15-18) (Figure 6A,B). Areas composed of poorly differentiated cells may be seen in the other subtypes or may occasionally constitute the entire tumor. In contrast to their soft tissue counterparts, mediastinal synovial sarcomas are more likely of monophasic (48%) and poorly differentiated types (~50%) as opposed to the biphasic type (<5%) (11,13) (Table 2).
Full table
Immunohistochemical and molecular features
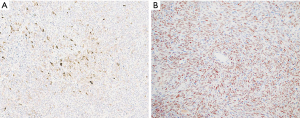
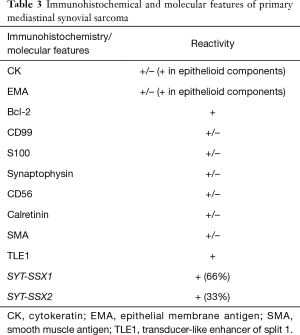
The diagnosis of synovial sarcoma is generally straightforward in the right clinical setting and typical tumor location. When arising in unusual sites, the tumors may be more difficult to diagnose and ancillary methods become indispensable. Until recently, the immunophenotype of synovial sarcoma was non-specific. Common markers that are expressed by these tumors, such as cytokeratins, epithelial membrane antigen (EMA) and bcl-2, are not specific to synovial sarcoma and are also expressed by a wide range of unrelated neoplasms (10,13,19-22). Likewise, antibodies that are commonly expressed by tumors in the differential diagnosis, including S100, synaptophysin or CD99 can also be reactive in synovial sarcoma. Epithelial differentiation in the form of cytokeratin or EMA reactivity is often focal in monophasic and poorly differentiated synovial sarcomas. Strong and diffuse expression of these markers is generally limited to the epithelioid elements in biphasic synovial sarcoma (10,22) (Figure 7A). The discovery that transducer-like enhancer of split 1 (TLE1) is overexpressed in synovial sarcomas has led to identification of TLE1 expression also on a protein level (23,24). Since then, TLE1 has emerged as a relatively sensitive and specific immunohistochemical marker for these tumors, with sensitivity rates ranging from 82% to 100% in various studies (19-21,25,26) (Figure 7B). On the other hand, TLE1 protein may also be detected in other mesenchymal neoplasms, including tumors in the differential diagnosis of synovial sarcoma, such as malignant peripheral nerve sheath tumor (MPNST) and solitary fibrous tumor (SFT) (20,23,25,26). One important aspect in this context is that TLE1 staining in synovial sarcoma is usually strong and diffuse while in other mesenchymal neoplasms is often less intense and more focal (Table 3). Synovial sarcomas are translocation-associated tumors. More than 95% of these tumors are characterized by a t(X;18)(p11;q11) translocation, leading to fusion of SS18 to one of the SSX genes. SS18-SSX1 is the most common fusion gene and can be identified in 2/3 of the cases while SS18-SSX2 can be found in the remainder (27-32) (Table 3). FISH or RT-PCR are common methods used to detect these rearrangements, enabling diagnostic confirmation in the majority of cases and representing the most specific and sensitive tool in the diagnosis of synovial sarcoma. Molecular analysis is of particular benefit in cases where diagnosis relies on small biopsy material or for tumors in unusual locations.
Full table
Differential diagnosis
The differential diagnosis for synovial sarcomas originating in the mediastinum is quite wide given the morphologic variability of tumors and broad spectrum of neoplastic conditions that can arise in the mediastinal space (Table 4). Monophasic synovial sarcomas must be distinguished from other spindle cell neoplasms. In the mediastinum, close attention should be paid to the tumor location within the individual compartments. For tumors in the posterior mediastinum, peripheral nerve sheath tumors should be considered. Both schwannoma and MPNST are composed of spindle cell proliferations that may be difficult to distinguish from synovial sarcoma based on tumor morphology alone. Schwannomas, however, are benign spindle cell tumors that should lack any features of malignancy, such as mitotic activity or areas of necrosis. MPNST and synovial sarcoma may share several morphological and immunohistochemical characteristics, including a malignant spindle cell proliferation and focal reactivity for cytokeratins and S100 protein. In this context, staining for TLE1 should be applied and usually demonstrates strong and diffuse reactivity in synovial sarcomas and absent or only weak non-specific staining in MPNST. SFT is another spindle cell neoplasm that can show strikingly similar tumor morphology as synovial sarcoma and that can arise in virtually any mediastinal compartment. These tumors are characterized by alternating zones of hyper and hypocellularity in a background of a variably fibrous stromal background. In contrast to synovial sarcoma, SFT is most commonly benign and devoid of malignant features. Furthermore, SFT is known to be positive for CD34 and STAT6 and negative for cytokeratins and TLE1 by immunohistochemistry and additionally harbors a characteristic NAB2-STAT6 gene fusion. For tumors arising in the anterior mediastinal compartment, thymic epithelial tumors with spindle cell morphology, i.e., spindle cell thymoma and spindle cell thymic carcinoma, need to be excluded. Spindle cell thymomas are low grade malignant neoplasms typically lacking mitotic activity while spindle cell thymic carcinomas show cytologic atypia and an increased mitotic rate. In cases of doubt, diffuse immunostaining with cytokeratin and p40 would favor a thymic spindle cell neoplasm while absent or focal keratin staining coupled with strong and diffuse reactivity for TLE1 would favor synovial sarcoma. Malignant mesothelioma may be confused with both monophasic and biphasic synovial sarcomas due to its similar morphological variability and growth patterns. In this context, sarcomatoid mesothelioma may closely mimic the monophasic type of synovial sarcoma whilst biphasic mesotheliomas and biphasic synovial sarcomas both contain spindle cell and epithelioid elements. However, mesotheliomas have a distinct disease distribution, typically affecting the pleural surfaces in a diffuse pattern, in contrast to synovial sarcoma which is usually a localized mass. Moreover, mesotheliomas are characterized by strong and diffuse staining for cytokeratin and other markers useful in the diagnosis of mesothelial lesions, for instance calretinin or WT-1. Reactivity for TLE1 is not a feature of these tumors. Another biphasic tumor that can occur in the anterior mediastinum is thymic carcinosarcoma. Unlike in synovial sarcoma, the glandular component in these tumors often shows overtly malignant features and contains poorly differentiated sarcomatoid elements that often lack the monotonous appearance of synovial sarcoma. Poorly differentiated synovial sarcoma may be difficult to distinguish from tumors in the primitive neuroectodermal tumor (PNET)/Ewing sarcoma group due to significant morphological and immunohistochemical overlap. In such cases, TLE1 should be applied and if positive would favor a diagnosis of synovial sarcoma; identification of the characteristic EWSR1 gene rearrangement in PNET/Ewing sarcoma by molecular methods offers another diagnostic tool in this context. Confirmatory molecular analysis for SS18 gene rearrangement can establish or support the diagnosis of synovial sarcoma in all cases where tumor morphology and immunophenotype fail to provide unequivocal separation from its closest mimics (Table 4). Finally, a metastatic process to the mediastinum from an extrathoracic primary should always be excluded based on detailed clinical and radiological correlation.

Full table
Treatment and prognosis
The treatment of choice for patients with mediastinal synovial sarcoma is complete surgical resection which is the only factor associated with improved survival (33). Neoadjuvant chemotherapy and radiotherapy should be considered in patients with unresectable non-metastatic disease followed by surgical intervention. Chemotherapy, especially high dose ifosfamide with or without doxorubicin can be administered in unresectable patients. Adjuvant chemotherapy and radiotherapy should be considered as part of a multimodality approach in all patients (5). Despite these multimodal treatment options, the prognosis for patients with mediastinal synovial sarcomas remains poor, with a median overall survival of 36 months and a 5-year overall survival rate of 35.7%, compared to a 5-year overall survival rate of 50–80% for extremity primaries (10-12,33-35). This is likely due to presentation at advanced tumor stage, large tumor size, difficulty of complete surgical resection due to involvement of vital anatomic structures, and high incidence of the poorly differentiated subtype. The type of gene fusion (SYT-SSX1 vs. SYT-SSX2) does not seem to have a significant effect on disease-specific survival among thoracic synovial sarcomas (13).
Comment
Synovial sarcomas originating in the mediastinum are rare neoplasms that may cause significant diagnostic difficulty due to the unusual tumor location. Although these tumors share many overlapping features with their soft tissue counterparts, they are characterized by slightly older age at presentation, bias towards the male gender, larger tumor size, advanced tumor stage and higher rate of the poorly differentiated subtype. These features contribute to the more aggressive clinical behavior and poor prognosis of these tumors. Completeness of resection appears to be the single most critical factor for improved survival and should be aggressively pursued in all patients with potentially resectable disease.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Saul Suster and David Suster) for the series “Mediastinal Sarcomas” published in Mediastinum. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med-20-19). The series “Mediastinal Sarcomas” was commissioned by the editorial office without any funding or sponsorship. AW serves as an unpaid editorial board member of Mediastinum from Oct 2019 to Sep 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cadman NL, Soule EH, Kelly PJ. Synovial sarcoma: an analysis of 34 tumors. Cancer 1965;18:613-27. [Crossref] [PubMed]
- Murray MR, Stout AP, Pogogeff IA. Synovial sarcoma and normal synovial tissue cultivated in vitro. Ann Surg 1944;120:843-51. [Crossref] [PubMed]
- Fisher C. Synovial sarcoma: ultrastructural and immunohistochemical features of epithelial differentiation in monophasic and biphasic tumors. Hum Pathol 1986;17:996-1008. [Crossref] [PubMed]
- Dickersin GR. Synovial sarcoma: a review and update, with emphasis on the ultrastructural characterization of the non-glandular component. Ultrastruct Pathol 1991;15:379-402. [Crossref] [PubMed]
- Eilber FC, Dry SM. Diagnosis and management of synovial sarcoma. J Surg Oncol 2008;97:314-20. [Crossref] [PubMed]
- Spurrell EL, Fisher C, Thomas JM, Judson IR. Prognostic factors in advanced synovial sarcoma: an analysis of 104 patients treated at the Royal Marsden Hospital. Ann Oncol 2005;16:437-44. [Crossref] [PubMed]
- Guillou L, Benhattar J, Bonichon F, et al. Histologic grade, but not SYT-SSX fusion type, is an important prognostic factor in patients with synovial sarcoma: a multicenter, retrospective analysis. J Clin Oncol 2004;22:4040-50. [Crossref] [PubMed]
- Goldblum JR, Folpe AL, Weiss SW. editors. Enzinger and Weiss’s Soft Tissue Tumors. 6th ed. Philadelphia: Elsevier Saunders, 2014:1052-70.
- Witkin GB, Miettinen M, Rosai J. A biphasic tumor of the mediastinum with features of synovial sarcoma. a report of four cases. Am J Surg Pathol 1989;13:490-9. [Crossref] [PubMed]
- Suster S, Moran CA. Primary synovial sarcomas of the mediastinum: a clinicopathologic, immunohistochemical, and ultrastructural study of 15 cases. Am J Surg Pathol 2005;29:569-78. [Crossref] [PubMed]
- Terra SB, Aesif SW, Maleszewski JJ, et al. Mediastinal synovial sarcoma: clinicopathologic analysis of 21 cases with molecular confirmation. Am J Surg Pathol 2018;42:761-6. [Crossref] [PubMed]
- Hartel PH, Fanburg-Smith JC, Frazier AA, et al. Primary pulmonary and mediastinal synovial sarcoma: a clinicopathologic study of 60 cases and comparison with five prior series. Mod Pathol 2007;20:760-9. [Crossref] [PubMed]
- Bégueret H, Galateau-Salle F, Guillou L, et al. Primary intrathoracic synovial sarcoma: a clinicopathologic study of 40 t(X;18)-positive cases from the French Sarcoma Group and the Mesopath Group. Am J Surg Pathol 2005;29:339-46. [Crossref] [PubMed]
- Gladish GW, Sabloff BM, Munden RF, et al. Primary thoracic sarcomas. Radiographics 2002;22:621-37. [Crossref] [PubMed]
- Chan JA, McMenamin ME, Fletcher CD. Synovial sarcoma in older patients: clinicopathological analysis of 32 cases with emphasis on unusual histological features. Histopathology 2003;43:72-83. [Crossref] [PubMed]
- Bergh P, Meis-Kindblom JM, Gherlinzoni F, et al. Synovial sarcoma: identification of low and high risk groups. Cancer 1999;85:2596-607. [Crossref] [PubMed]
- de Silva MV, McMahon AD, Paterson L, et al. Identification of poorly differentiated synovial sarcoma: a comparison of clinicopathological and cytogenetic features with those of typical synovial sarcoma. Histopathology 2003;43:220-30. [Crossref] [PubMed]
- van de Rijn M, Barr FG, Xiong QB, et al. Poorly differentiated synovial sarcoma: an analysis of clinical, pathologic, and molecular genetic features. Am J Surg Pathol 1999;23:106-12. [Crossref] [PubMed]
- Pelmus M, Guillou L, Hostein I, et al. Monophasic fibrous and poorly differentiated synovial sarcoma: immunohistochemical reassessment of 60 t(X;18)(SYT-SSX)-positive cases. Am J Surg Pathol 2002;26:1434-40. [Crossref] [PubMed]
- Jagdis A, Rubin BP, Tubbs RR, et al. Prospective evaluation of TLE1 as a diagnostic immunohistochemical marker in synovial sarcoma. Am J Surg Pathol 2009;33:1743-51. [Crossref] [PubMed]
- Knösel T, Heretsch S, Altendorf-Hofmann A, et al. TLE1 is a robust diagnostic biomarker for synovial sarcomas and correlates with t(X;18): analysis of 319 cases. Eur J Cancer 2010;46:1170-6. [Crossref] [PubMed]
- Miettinen M, Limon J, Niezabitowski A, et al. Patterns of keratin polypeptides in 110 biphasic, monophasic, and poorly differentiated synovial sarcomas. Virchows Arch 2000;437:275-83. [Crossref] [PubMed]
- Terry J, Saito T, Subramanian S, et al. TLE1 as a diagnostic immunohistochemical marker for synovial sarcoma emerging from gene expression profiling studies. Am J Surg Pathol 2007;31:240-6. [Crossref] [PubMed]
- Baird K, Davis S, Antonescu CR, et al. Gene expression profiling of human sarcomas: insights into sarcoma biology. Cancer Res 2005;65:9226-35. [Crossref] [PubMed]
- Kosemehmetoglu K, Vrana JA, Folpe AL. TLE1 expression is not specific for synovial sarcoma: a whole section study of 163 soft tissue and bone neoplasms. Mod Pathol 2009;22:872-8. [Crossref] [PubMed]
- Foo WC, Cruise MW, Wick MR, et al. Immunohistochemical staining for TLE1 distinguishes synovial sarcoma from histologic mimics. Am J Clin Pathol 2011;135:839-44. [Crossref] [PubMed]
- Ladanyi M, Antonescu CR, Leung DH, et al. Impact of SYT-SSX fusion type on the clinical behavior of synovial sarcoma: a multi-institutional retrospective study of 243 patients. Cancer Res 2002;62:135-40. [PubMed]
- Kawai A, Woodruff J, Healey JH, et al. SYT-SSX gene fusion as a determinant of morphology and prognosis in synovial sarcoma. N Engl J Med 1998;338:153-60. [Crossref] [PubMed]
- Lewis JJ, Antonescu CR, Leung DH, et al. Synovial sarcoma: a multivariate analysis of prognostic factors in 112 patients with primary localized tumors of the extremity. J Clin Oncol 2000;18:2087-94. [Crossref] [PubMed]
- Brodin B, Haslam K, Yang K, et al. Cloning and characterization of spliced fusion transcript variants of synovial sarcoma: SYT/SSX4, SYT/SSX4v, and SYT/SSX2v. Possible regulatory role of the fusion gene product in wild type SYT expression. Gene 2001;268:173-82. [Crossref] [PubMed]
- Sandberg AA, Bridge JA. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors. Synovial sarcoma. Cancer Genet Cytogenet 2002;133:1-23. [Crossref] [PubMed]
- dos Santos NR, de Bruijn DR, van Kessel AG. Molecular mechanisms underlying human synovial sarcoma development. Genes Chromosomes Cancer 2001;30:1-14. [Crossref] [PubMed]
- Salah S, Salem A. Primary synovial sarcomas of the mediastinum: a systematic review and pooled analysis of the published literature. ISRN Oncol 2014;2014:412527 [Crossref] [PubMed]
- Deshmukh R, Mankin HJ, Singer S. Synovial sarcoma: the importance of size and location for survival. Clin Orthop Relat Res 2004;155-61. [Crossref] [PubMed]
- Ulmer C, Kettelhack C, Tunn PU, et al. Synovial sarcoma of the extremities. Results of surgical and multimodal therapy. Chirurg 2003;74:370-4. [Crossref] [PubMed]
Cite this article as: Syred K, Weissferdt A. Primary mediastinal synovial sarcomas. Mediastinum 2020;4:13.


