
Paediatric mesenchymal tumors of the mediastinum
Introduction
The mediastinum is compartmentalized into four—the anterior, middle, posterior and superior, each having its own components (1). Also, the different mediastinal lesions vary in the tumor biology with respect to the age of presentation (2). The mesenchymal tumors form only 2% of all mediastinal neoplasms (3), however, along with the cysts, they form the major component of mediastinal tumors in the children and adolescents (2). Tumors of the posterior mediastinum comprise of 40% of all childhood mesenchymal tumors of the mediastinum most of which are of neurogenic origin (4). Among the mesenchymal tumors, benign lesions form the major bulk of which the lipomas and lymphangiomas predominate in the paediatric age group (2).
Mesenchymal tumors of the adipocytic lineage
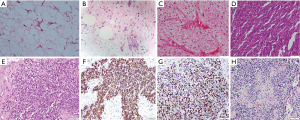
The various entities encompassing this category include the benign entities comprising of the lipomas and the lipoblastomas and the malignant counterpart namely liposarcomas. Of these, the lipomas can occur at any age and may involve any part of the mediastinum (Figure 1A). The lipoblastomas are exclusively the tumors of infancy and is rarely seen beyond 3 years of age (5,6). They are locally invasive and are prone to local recurrence but do not metastasize. They are known to occur in the extremities and the axillary and supraclavicular regions. A mediastinal location is extremely rare among the lipoblastomas (Figure 1B) (7). Even though benign, lipoblastomas are known to recur and may involve the spinal canal at times (8). Among the malignant tumors, mediastinal origin is found in only 11% of all childhood liposarcomas (5) which may be associated with the Li-Fraumeni syndrome (9). Most of them are of the myxoid liposarcoma (9) and may also arise within the thymus gland (Figure 1C). Even though all described histopathological variants of liposarcomas has been described in the mediastinum, the pleomorphic myxoid liposarcoma variant has been seen to have a predilection for the younger age group of 13–20 years (8). Histopathologically, besides the conventional areas of low grade myxoid liposarcoma, areas with pleomorphic lipoblasts with increased mitotic activity are present. These tumors do not possess the characteristic translocations and tend to have an aggressive clinical course, nevertheless surgical resection remains the treatment of choice. The liposarcomas in children have to be distinguished from lipoblastomatosis which mostly occurs before the age of two years whereas liposarcomas are not seen in children less than 3 years (10). Microscopically, benign lipoblastomatosis, especially the diffuse type closely resemble myxoid liposarcoma (Figure 1D). Both of them have a prominent plexiform vascular network with interspersed lipoblasts. The lipoblastomatosis have small lobules of adipocytes in different stages of maturation. However, there is absence of mitotic activity, bizarre or giant cells and no mucoid or microcystic appearance, features which are frequently present in myxoid liposarcoma (Figure 1E) (10).
Tumors of smooth/skeletal muscle origin
The tumors of smooth muscle origin comprise of leiomyoma and leiomyosarcoma (Figure 1F) which are extremely rare in the paediatric age group. The skeletal muscle tumors in the mediastinum include rhabdomyoma, rhabdomyosarcoma, rhabdomyosarcoma as a component of Triton tumor or as a somatic-type malignancy in germ cell tumours. Of these the rhabdomyomas are benign lesions thought to be hamartomas arising from the heart in children with tuberous sclerosis (11). These are extremely rare in the mediastinum occurring almost exclusively in children. Primary mediastinal rhabdomyosarcomas (Figure 1G) are very rare and are usually found as part of the heterologous component of the other lesions (3,11). These tumors occur across all paediatric age groups and are usually asymptomatic. They commonly originate from the pleura or the diaphragm. Complete surgical resection without any residual disease is associated with good prognosis. However, achievement of negative margins may be difficult especially in tumors encasing vital organs (5).
Tumors of cartilage origin
Extraskeletal mesenchymal chondrosarcomas form a common entity of this subgroup in the posterior mediastinum of the children between 11–20 years of age. Radiology reveals a soft tissue mass with punctate calcification with a differential diagnosis of neuroblastoma (5). Histologically these tumors are biphasic comprising of mature and immature cartilaginous elements and spindle cell population (Figure 1H). Nevertheless it has to be differentiated from a myriad of differentials including hemangiopericytoma, solitary fibrous tumor, monophasic synovial sarcoma and myxoid liposarcoma (12).
Tumors of neurogenic origin
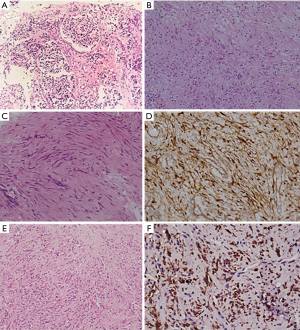
Neurogenic tumors, especially the malignant ones predominate in the paediatric age group (13,14). The tumors in this category comprise of those of schwannian derivation which are common in adults and from the autonomic nervous system which predominate in the children (11). Tumors originating from the sympathetic nervous system are usually paraspinal in location with erosion into the ribs and the spinal column. The postero-superior mediastinum houses the infiltrative neuroblastomas which are commonly seen in children less than 5 years of age and have poor prognosis (Figure 2A) (4). Children with this tumor are symptomatic with presence of fever, cough, vomiting, diarrhoea and other paraneoplastic symptoms (14). When found in adults, they are usually of thymic origin. The neuroblastomas are associated with MYCN gene amplifications and mutations of ALK, PHOX2B, and ATRX have also been described. They have also shown in addition expression of neuron-specific enolase (NSE) and GATA3 (3). The other neurogenic lesions of the peripheral nerves such as the schwannomas, neurofibromas and ganglioneuromas (Figure 2B) occur in older children. The tumors of neuroectodermal origin have also been reported in the mediastinum of which the rare entity of melanotic neuroectodermal tumor of infancy is found in the paediatric age group. They are usually located in the jaws and case reports of mediastinal location exist. These tumors histologically comprise of biphasic population of small cells which are immunoreactive for neuronal/neuroendocrine (synaptophysin and chromogranin) and the larger cells which show immunoreactivity for EMA, cytokeratin and HMB45 (11).
Tumors of fibroblastic/myofibroblastic origin
Tumors of fibroblastic/myofibroblastic origin generally have a predilection for the skin and superficial soft tissues and are very rare in the mediastinal location. Of the few cases which occur in the mediastinum, the aggressive fibromatosis (Figure 2C,D) is seen in the younger population and has a female preponderance (8). Similarly, the inflammatory myofibroblastic tumor is seen in children with a female preponderance in the mediastinal ones (Figure 2E,F). Histologically they are composed of myofibroblasts with admixed inflammatory infiltrate comprising of lymphocytes, plasma cells and eosinophils and may exhibit anaplastic lymphoma kinase (ALK) gene rearrangement. Such tumors are surgically resected and may be amenable to targeted therapy for ALK rearrangement, although the benefits of this therapy has not been proven for the mediastinal lesions (8). The extrapleural solitary fibrous tumor, rarely seen in children, behave more aggressively than their counterparts in the extremities (9).
Tumors of vascular origin
The benign or intermediate-grade tumors of vascular origin comprise of the hemangiomas, lymphangiomas and the hemangioendotheliomas. The hemangiomas have an equal distribution among the paediatric and adult population and up to 50% of these cases are asymptomatic. The lymphangiomas are common in the paediatric age group and very rare in the adults. They are generally malformations of the lymphatic channels with large lesions presenting as dyspnoea. They are curable after surgical resection with very few recurrences (11). Among the intermediate-grade tumors, the Kaposiform Hemangioendothelioma (Figure 3A) is almost exclusively seen in the infants (11). They are often associated with thrombocytopenic consumptive coagulopathy which is the cause of its fatal outcome. Histologically they mimic their soft tissue counterparts and are Human Herpes Virus-8 negative.
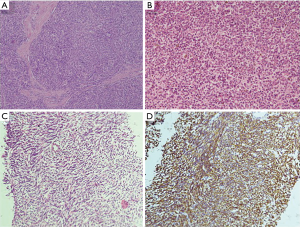
Hemangiopericytomas (HPC) which are tumors of pericytic origin are also rare tumors seen in children forming <10% of all reported cases. It usually arises in the head and neck region, lower extremities or the retroperitoneum and its location in the mediastinum is rare. They may be asymptomatic or may present with symptoms of mass effect. The paediatric HPC may be clinically distinguished into two subtypes namely, infantile type (seen in children <1 year of age) or the adult type (children older than 1 year). The treatment for both the subtypes differ as the infantile type may regress with surgical therapy alone and even chemosensitive whereas the adult type is considered to have poorer prognosis (15).
Tumors of uncertain origin/small round cell tumors
The Ewings sarcoma, also known as the Askin tumor if it arises from the chest wall, is rare in the mediastinum. However, it is the commonest round cell neoplasm among the paediatric age group in this location. The primary mediastinal extraskeletal Ewings sarcoma are rare and warrant an exclusion of a vertebral or chest wall origin before labelling them as such (11). Similar to its counterparts in the other locations, demonstration of the translocation involving the EWSR1 gene is necessary for its confirmation (3). The differential diagnosis of this lesion is broad and includes Ewings sarcoma (Figure 3B) with variant translocations, synovial sarcomas and other small blue round cell tumours especially the T lymphoblastic lymphomas, both of which show CD99 positivity (11). The other immunohistochemical markers for lymphoma and the absence of the characteristic EWSR translocation of Ewing’s help in the differentiation between the two. The other paediatric tumors falling into this category include few cases of synovial sarcoma and case reports of epithelioid sarcoma (11). The synovial sarcomas (Figure 3C,D) are predominantly located in the anterior mediastinum and are associated with the similar characteristic translocation as found in their soft tissue counterparts.
Conclusions
Mesenchymal tumors of the mediastinum are rare neoplasms and their incidence in the paediatric population is even rarer except some which are exclusively seen in this age group. The primary treatment modality for all these neoplasms is surgical resection. A complete resection is potentially curative for most neoplasms. However, the complexities of the mediastinal anatomy make complete resection difficult at times with residual disease post-surgery leading to recurrences. Few lesions are also amenable to chemo and radiotherapy. This review was aimed to bring together a comprehensive collection of a rare group of mediastinal lesions.
Acknowledgments
Dr. Deepali Jain, Additional Professor, Department of Pathology, All India Institute of Medical Sciences, New Delhi, India.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Deepali Jain) for the Special Series “Pediatric Mediastinal Tumors” published in Mediastinum. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2020.01.03). The Special Series “Pediatric Mediastinal Tumors” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interests to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Grosfeld JL, Skinner MA, Rescorla FJ, et al. Mediastinal tumors in children: experience with 196 cases. Ann Surg Oncol 1994;1:121-7. [Crossref] [PubMed]
- Liu T, Al-Kzayer LFY, Xie X, et al. Mediastinal lesions across the age spectrum: a clinicopathological comparison between pediatric and adult patients. Oncotarget 2017;8:59845-53. [Crossref] [PubMed]
- den Bakker M, Marx A, Ströbel P. The pathology of mesenchymal tumors of the mediastinum. Mediastinum 2018;2:42. [Crossref]
- Buckley JA, Vaughn DD, Jabra AA, et al. CT Evaluation of Mediastinal Masses in Children: Spectrum of Disease with Pathologic Correlation. Crit Rev Diagn Imaging 1998;39:365-92. [Crossref] [PubMed]
- Billmire DF. Germ cell, mesenchymal, and thymic tumors of the mediastinum. Semin Pediatr Surg 1999;8:85-91. [Crossref] [PubMed]
- Moaath A, Raed E, Mohammad R, et al. Lipoblastoma: A Rare Mediastinal Tumor. Ann Thorac Surg 2009;88:1695-7. [Crossref] [PubMed]
- Ching AS, Lee SF, Chan YL. Diagnosing paediatric mediastinal lipoblastoma using ultrasound-guided percutaneous needle biopsy: Review and report. Clin Imaging 2002;26:23-6. [Crossref] [PubMed]
- den Bakker MA, Marx A, Mukai K, et al. Mesenchymal tumours of the mediastinum—part I. Virchows Archiv 2015;467:487-500. [Crossref] [PubMed]
- Fletcher CDM. Bridge JA, Hogendoorn PCW, et al. World Health Organization classification of tumours of soft tissue and bone. 4th edition. Lyon, WHO, 2013.
- Plukker JT, Joosten HJ, Rensing JB, et al. Primary liposarcoma of the mediastinum in a child. J Surg Oncol 1988;37:257-63. [Crossref] [PubMed]
- den Bakker MA, Marx A, Mukai K, et al. Mesenchymal tumours of the mediastinum—part II. Virchows Archiv 2015;467:501-17. [Crossref] [PubMed]
- Suster S, Moran CA. Malignant cartilaginous tumors of the mediastinum: clinicopathological study of six cases presenting as extraskeletal soft tissue masses. Hum Pathol 1997;28:588-94. [Crossref] [PubMed]
- Takeda S, Miyoshi S, Akashi A, et al. Clinical spectrum of primary mediastinal tumors: A comparison of adult and pediatric populations at a single Japanese institution. J Surg Oncol 2003;83:24-30. [Crossref] [PubMed]
- Silverman NA, Sabiston DC Jr. Primary tumors and cysts of the mediastinum. Curr Probl Cancer 1977;2:1-55. [Crossref] [PubMed]
- Horikawa-Kyo Y, Tanaka T, Tanano H, et al. Mediastinal hemangiopericytoma. Pediatr Blood Cancer 2009;53:206-7. [Crossref] [PubMed]
Cite this article as: Guleria P, Barwad A. Paediatric mesenchymal tumors of the mediastinum. Mediastinum 2020;4:11.


