Mediastinal soft tissue sarcoma: dark sides and future lights
Soft tissue sarcomas (STS), are rare tumors of mesenchymal origin, account for 1% of all cancers (1). Even though these tumours may arise anywhere in the body, the most common primary sites are extremities and abdomen (2). STS of the mediastinum are very rare malignancies representing only <10% of primary mediastinal tumours and <1% of all STS (3-5).
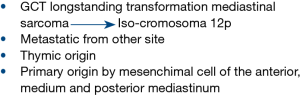
The mediastinum is a complex anatomic space in which organ and major blood vessels reside with surrounding soft tissue elements. Mediastinal malignancies include epithelial tumours of thymic origin (thymoma and thymic carcinoma), lymphomas, germ cell tumours (GCT), metastasis arising from other sites and primary tumours originating from mesenchimal cell of the anterior, medium and posterior mediastinum. Moreover, recent findings confirmed also that spectrum and frequency of mediastinal lesions depends properly on mediastinal compartment, which identification may be helpful for the initial differential diagnosis of a mediastinal lesion (6).
To date, only few individual case report and small series of patients with STS of mediastinum have been reported (7,8). Owing to the complex anatomic features of mediastinum, often the mesenchymal tumours are detected at advanced stage, when vascular and lung compression symptoms arise.
Pathological definition of mediastinal sarcoma (MS) is often challenging and requires high expertise. Particular attention is needed especially in young male patients, since it must be considered that sarcomatous tissue may remain as the residual component or the metastatic vestige of burnt-out primary mediastinal GCT. The identification of isochromosome 12p could be helpful to confirm the origin of MS (rhabdomyosarcoma and angiosarcoma subtype) from a GCT (9,10).
Pathologists should also take into account that sarcomatous area may be part of thymic-sarcomatoid carcinoma or pseudo-sarcomatous stroma in thymoma (11,12) (Figure 1).
Pachter et al., in 1963, published a systematic review of the mediastinal soft tissue pathology (13), followed by the most recent two-part review by den Bakker et al., based on the 2013 WHO classification of soft tissue tumours and the 2015 WHO classification of tumours of lung, pleura, thymus and heart (Figure 2) (14,15). The histo-pathological variety of MS has been recently enriched by the review of You-Li Wo et al., with the description of the very rare entity of follicular dendritic cell sarcoma (FDCS) (16).
Complexity, rarity and literature data fragmentation make very difficult to define shared treatment strategy for the management of MS, therefore the study published by Engelhardt et al. is a reference and starting point for the knowledge of treatment approaches and survival outcomes of MS patients.
Analysing data from the National Cancer Database (NCDB), one of the largest cancer registries worldwide, the authors carried out the description of 976 patients affected by MS correlating the survival outcomes with treatment modality (no treatment, radiation and/or chemotherapy, surgery only, surgery and radiation, surgery and chemotherapy, and surgery and chemoradiation); surgery characteristics (R0, R1, R2, or unknown); tumour size (10 centimeters); histology subtype (hemangiosarcoma, leiomyosarcoma, synovial sarcoma, sarcoma not otherwise specified, malignant peripheral nerve sheath tumour and other); grade of tumour, patients and hospital-level characteristics (17).
The authors generated relevant points of interest that should be commented on.
First of all, as previously reported, the analysis confirmed a male predominance of disease and a poor prognosis with a five-year overall survival (OS) of only 14.8% in the entire cohort, but surprisingly, emangiosarcoma, rarely diagnosed in mediastinum (18), was reported as the predominant histotype.
Secondary, in this setting, this is the first study to examine the association between OS and treating facility characteristics or patient-specific social determinants of health, highlighting that factors like facility location, academic status, graduate medical education programs, educational levels of the patient and the insurance status, may impact on the survival outcome.
At last very remarkable is the authors’ effort to correlate the delivered treatment to the survival outcome even in a highly heterogeneous cohort. As already acknowledged for sarcoma of other anatomic location and reported in previous case series of MS (19), surgery, also in this study, remains the primary therapeutic strategy, however, in this series, less than half of patients underwent surgical resection, likely because MS are frequently detected at advanced stage with invasion of vital structures, precluding resection. This anatomical peculiarity, however, should explain why the debulking surgery impacts positively on OS especially when followed by radiation therapy, differently from the sarcoma of other anatomic site, where the application of debulking surgery is still controversial.
Moreover, intriguingly, the radiotherapy was performed with the same rate in both patients with RO resection (28%) and with positive margin R1-R2 (30%), achieving the best 5 years survival (P=0.002) in patients who received R0 surgical resection and radiotherapy. Worthy of consideration is the uncertain role of chemotherapy, mainly because of the pathological heterogeneity, the lack of information about the delivered cytotoxic drugs, the inclusion of histo-type not chemo-sensitive and the absence of histology driven chemotherapy due to the time of data collection.
Conclusions
This the largest cohort of patients with MS ever reported, that identifies surgery as the cornerstone of therapy. Radiation therapy may offer some survival advantages, while the role of chemotherapy remains unclear. International reference centers with high expertise in the field of rare cancers should plan prospective registered database on the specific topic of MS in order to improve the current knowledge and provide a worldwide multidisciplinary consensus for the best treatment strategies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Dr. Luigi Ventura (Thoracic Surgery, Surgical Unit, Department of Medicine and Surgery, University Hospital of Parma, Parma, Italy).
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2020.03.02). GP serves as an unpaid editorial board member of Mediastinum from Oct 2019 to Sep 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Tiwari R, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin 2004;54:8-29. [Crossref] [PubMed]
- Clark MA, Fisher C, Judson I, et al. Soft-tissue sarcomas in adults. N Engl J Med 2005;353:701-11. [Crossref] [PubMed]
- Macchiarini P, Ostertag H. Uncommon primary mediastinal tumours. Lancet Oncol 2004;5:107-18. [Crossref] [PubMed]
- Toro JR, Travis LB, Wu HJ, et al. Incidence pat- terns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978-2001: an analysis of 26,758 cases. Int J Cancer 2006;119:2922-30. [Crossref] [PubMed]
- Burt M, Ihde JK, Hajdu SI, et al. Primary sarcomas of the mediastinum: results of therapy. J Thorac Cardiovasc Surg 1998;115:671-80. [Crossref] [PubMed]
- Roden AC, Fang W, Shen Y, et al. Distribution of Mediastinal Lesions Across Multi-Institutional, International, Radiology Databases. J Thorac Oncol 2019; [Epub ahead of print]. [PubMed]
- Burt M, Ihde JK, Hajdu SI, et al. Primary sarcomas of the mediastinum: results of therapy. J Thorac Cardiovasc Surg 1998;115:671-80. [Crossref] [PubMed]
- Coindre JM, Terrier P, Bui NB, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol 1996;14:869-77. [Crossref] [PubMed]
- Motzer RJ, Amsterdam A, Prieto V, et al. Teratoma with malignant transformation: diverse malignant histologies arising in men with germ cell tumors. J Urol 1998;159:133-8. [Crossref] [PubMed]
- Wehle D, Yonescu R, Long PP, Gala N, Epstein J, Griffin CA. Fluorescence in situ hybridization of 12p in germ cell tumors using a bacterial artificial chromosome clone 12p probe on paraffin-embedded tissue: clinical test validation. Cancer Genet Cytogenet 2008;183:99-104. [Crossref] [PubMed]
- Eimoto T, Kitaoka M, Ogawa H, et al. Thymic sarcomatoid carcinoma with skeletal muscle differentiation: report of two cases, one with cytogenetic analysis. Histopathology 2002;40:46-57. [Crossref] [PubMed]
- Suster S, Moran CA, Chan JK. Thymoma with pseudosarcomatous stroma: report of an unusual histologic variant of thymic epithelial neoplasm that may simulate carcinosarcoma. Am J Surg Pathol 1997;21:1316-23. [Crossref] [PubMed]
- Pachter MR, Lattes R. Mesenchymal tumors of the mediastinum.I. Tumors of fibrous tissue, adipose tissue, smooth muscle, and striated muscle. Cancer 1963;16:74-94. [Crossref] [PubMed]
- den Bakker MA, Marx A, Mukai K, et al. Mesenchymal tumours of the mediastinum--part I. Virchows Arch 2015;467:487-500. [Crossref] [PubMed]
- den Bakker MA, Marx A, Mukai K, et al. Mesenchymal tumours of the mediastinum--part II. Virchows Arch 2015;467:501-17. [Crossref] [PubMed]
- Wu YL, Wu F, Xu CP, et al. Mediastinal follicular dendritic cell sarcoma: a rare, potentially under-recognized, and often misdiagnosed disease. Diagn Pathol 2019;14:5. [Crossref] [PubMed]
- Engelhardt KE, DeCamp MM, Yang AD, et al. Treatment Approaches and Outcomes for Primary Mediastinal Sarcoma: Analysis of 976 Patients. Ann Thorac Surg 2018;106:333-9. [Crossref] [PubMed]
- Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683-90. [Crossref] [PubMed]
- Luo DX, Huang MJ, Xiong B, et al. Primary mediastinal sarcoma: surgical outcomes of 21 cases. Interact Cardiovasc Thorac Surg 2013;17:982-6. [Crossref] [PubMed]
Cite this article as: Palmieri G, Tortora M, Parola S, Picozzi F, Ottaviano M. Mediastinal soft tissue sarcoma: dark sides and future lights. Mediastinum 2020;4:9.