
Invasive mediastinal staging by endosonography or video-assisted mediastinoscopy in PET-CT clinical N1 non-small cell lung cancer
Introduction
In patients with suspected non-small lung cancer and enlarged or FDG-avid hilar or intrapulmonary lymph nodes (clinical N1-disease, cN1-disease) the risk of unforeseen positive mediastinal nodes (N2-disease) at resection is estimated between 20% and 30% (1-4).
Current guidelines recommend invasive preoperative mediastinal staging in these cN1 patients, i.e., video-assisted mediastinoscopy (VAM) or endosonography (5,6). However, no recommendation is made which of both techniques should be preferred. Furthermore, the recommendation to perform a confirmatory mediastinoscopy after a negative endosonography is debated. The argument against a confirmatory mediastinoscopy is based on studies with less than 10% N1 patients, the large majority being cN2 on imaging (7,8).
Two multicenter prospective studies reported on the sensitivity, negative predictive value (NPV) and accuracy of either endosonography or VAM in a well-defined group of cN1 patients (9,10).
Methods
Both studies were investigator-initiated non-randomized multicenter prospective observational cohort studies performing endosonography in the first study and VAM in the second study in consecutive patients with operable and resectable non-small cell lung cancer (NSCLC) staged cT1-T3N1M0 based on PET-CT. The endosonography study had three participating centers including patients between 2009 and 2013, the mediastinoscopy study nine centers including consecutive cases between 2014 and 2017.
In both studies, patients were eligible if they had medical operable, surgical resectable (suspected) NSCLC and cN1 disease based on integrated FDG-PET-CT. This included enlarged lymph nodes (defined as ≥10 mm in largest short axis on CT) or FDG-PET positive lymph node in a N1 position in accordance to the IASLC lymph node map (i.e., lymph node station 10 to 14) (11). Lymph nodes were considered positive on FDG-PET if the FDG uptake was higher than the background uptake in the mediastinal blood pool. Clinical T stages T1, T2 and selected T3 (i.e., intraparenchymal tumor >7 cm, T3 invading the chest wall, or T3 based on additional nodule in the lobe of the primary tumor) tumors were allowed (TNM 7th edition). Patients with former therapy for lung cancer, irresectable disease, cT4 or a central tumor staged cT3 (i.e., invasion of mediastinal pleura, invasion of phrenic nerve or parietal pericardium, tumor in the main bronchus less than 2 cm from the main carina), enlarged or FDG-positive mediastinal nodes, distant metastases (cM1) or previous EBUS assessment of mediastinal nodes were excluded from both studies.
Endosonography
Endosonography of the mediastinum was performed using a dedicated ultrasound bronchoscope or combined ultrasound bronchoscope and esophageal endoscope. Transbronchial and esophageal procedures were performed during a single session by the same bronchoscopist in each center. The minimal requirement was to explore stations 2L-4L-7 in case of a left-sided upper lobe primary tumor, stations 4L-7-8-9 in case of a left-sided lower lobe primary tumor, or stations 2R-4R-7 in case of a right-sided primary tumor. Nodes larger than 5 mm in short axis were sampled minimal two times under real-time ultrasound guidance with a 22-gauge needle, labeled according to the IASLC lymph node map and sent for pathological examination.
Cervical VAM
VAM was performed after negative endoscopy in the first study and was the primary invasive mediastinal staging procedure in the second study.
VAM was performed in a dedicated thoracic operating room by experienced thoracic surgeons. In accordance to ESTS guidelines, all accessible mediastinal nodes were to be sampled, the minimal stations being 4L-4R-7. Video-assisted mediastinoscopic lymphadenectomy (VAMLA) was allowed. Its indication was depending of surgeon’s discretion. In a standard VAM, lymph nodes at the different stations are assessed by sampling and not necessarily removed completely. During a VAMLA, typically the subcarinal nodes and right paratracheal nodes are removed completely with the surrounding fat and the left paratracheal nodes are removed separately with respect for the left recurrent nerve. Video-assisted thoracic surgery (VATS) or parasternal mediastinoscopy were not considered part of the preoperative mediastinal staging.
Surgical resection
If invasive mediastinal staging was negative, the patient underwent primary surgery with resection and surgical verification by transthoracic mediastinal lymphadenectomy. Resection could be performed by VATS or thoracotomy. The ESTS guidelines on perioperative systematic nodal dissection were to be followed (12).
Endpoints
The primary endpoint was sensitivity to detect mediastinal nodal involvement (N2-disease) by the invasive mediastinal staging technique, being endosonography in the first study and by VAM or VAMLA [VAM(LA)] in the second study. Sensitivity was defined as the proportion of patients with positive mediastinal staging by the respective invasive mediastinal staging technique over all the patients with mediastinal nodal disease.
Surgical resection with lymphadenectomy (by thoracotomy or VATS) was considered the reference standard for patients without mediastinal nodal disease after the invasive mediastinal staging technique. Secondary endpoints were NPV and assessment of the prevalence of N2/3 disease.
Statistics
Sensitivity, prevalence and NPV were calculated on an intent-to-treat basis for all included patients. For patients with missing reference standard (i.e., no primary surgery after negative invasive staging), a multiple imputation analysis was used to obtain estimates for sensitivity, prevalence and NPV on all subjects. P values smaller than 0.05 were considered significant.
Results
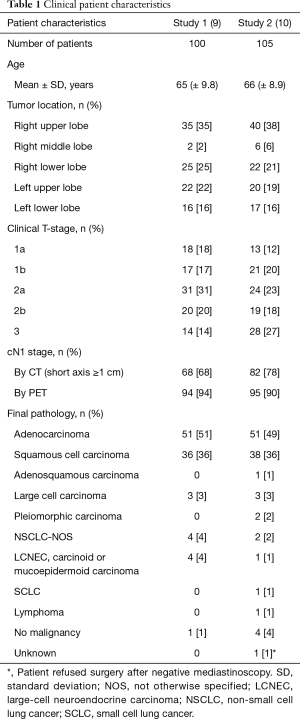
Between 2009 and 2013, 100 consecutive patients were included in the first study on endosonography (9) and between 2014 and 2017 105 patients in the second study with VAM(LA) (10). The clinical patient characteristics of both studies are shown in Table 1.
Full table
Study 1: endosonography
With endosonography a median of 2.1 mediastinal nodes were biopsied. Endosonography (n=100) detected mediastinal nodal disease (N2) in 10% (n=10) of patients. In an additional 14 patients N2 was found after mediastinoscopy (n=7/75), immediate resection (n=1/10) or resection after negative mediastinoscopy (n= 6/67).
These missed mediastinal metastases were single station N2 disease in 12 patients and multi-station N2 disease in 2 patients. The missed mediastinal metastases were located in station 7 (n=5), station 4R (n=4), station 2R (n=1), station 4L (n=2), station 8 (n=1), and station 5 (n=3). In 6 patients with missed mediastinal nodes, these nodes were not found by the additional mediastinoscopy but at resection: station 7 (n=1), station 4R (n=1), station 4L (n=1), and station 5 (n=3). Six patients did not undergo a resection (i.e., reference standard), 5 after a negative endosonography and one after negative endosonography and negative VAM.
The sensitivity to detect mediastinal positive nodes in cN1 lung cancer was 38% (95% CI: 18–57%) with endosonography alone according to an intent-to-treat analysis after correction with multiple imputation for the 6 patients without reference standard (primary surgery). The sensitivity of invasive staging increased to 73% (95% CI: 55–91%) by adding a confirmation mediastinoscopy if endosonography was negative (9). The NPV for endosonography was 81% (95% CI: 71–91%) and for endosonography plus cervical mediastinoscopy 91% (95% CI: 83–98%). The overall prevalence of mediastinal nodal disease was 24%. The estimated number needed to treat (NNT) based on multiple imputation data was 10 patients undergoing an extra cervical mediastinoscopy to identify one extra case of mediastinal nodal disease after a negative endosonography.
Study 2: VAM(LA)
The mean number of biopsied lymph nodes was 3.9. Positive mediastinal nodes were identified by VAM(LA) in 20 of 105 patients. In 31% (n=33) the procedure was labeled as a VAMLA. In two patients, the procedure was aborted before any lymph node was assessed. In one patient the mediastinoscope could not be introduced due to severe kyphosis. In the other, early cessation was necessary due to tracheomalacia and ventilatory problems during the procedure. A severe adverse event related to VAM was reported in 4 patients (4%): one bleeding of less than 200 cc, one uncomplicated wound infection and two cases of transient recurrent nerve paralysis.
Eighty-three patients underwent primary surgery. In seven patients, positive mediastinal nodes were found at resection. One of these was a patient where the mediastinoscopy was aborted prematurely. The missed mediastinal metastases were single-level N2 disease in five patients, and multi-level N2 disease in two patients. They were located in station 7 (n=5), station 4R (n=2) and station 6 (n=2).
For two patients the reference standard after negative VAM(LA), i.e., primary surgery with assessment of mediastinal nodes, was missing. Out of 83 patients with a negative test result after successful (not aborted) VAM(LA), 6 had pN2/N3 at resection.
According to an intent-to-treat analysis with multiple imputation the, sensitivity of VAM(LA) was 73% (95% CI: 54–86%), NPV 92% (95% CI: 83–97%) and the prevalence of mediastinal nodal metastases was 26% (95% CI: 18–35%) overall (Table 2).

Full table
Discussion
Few reports in the literature evaluated the final pathological stage distribution of patients with resectable and operable NSCLC with clinical stage cN1. Hishida et al. and Watanabe et al. reported that 30–37% of patients with cN1 based on CT alone had positive mediastinal nodes after mediastinoscopy (1,2). Kim et al. reported that 19% of 99 patients with cN1 after imaging including FDG-PET, were found to have pathologic N2 disease at pulmonary resection with mediastinal lymph node dissection (4). Mizuno et al. found a prevalence of 26% of N2-disease in patients with radiological diagnosed cN1 NSCLC in a retrospective study with 164 patients (13).
In our two prospective studies on invasive mediastinal staging, with in total more than 200 patients, we found that one in four patients with cN1 lung cancer and staged by PET-CT eventually had N2-disease after invasive staging and/or resection. The sensitivity of VAM(LA) to detect positive mediastinal nodes in these patients was 73%. Endosonography alone reached a sensitivity of 38%. NPV of endosonography was 81%. Therefore, a patient with a negative endosonography had a probability of 19% of a positive surgical result with respect to mediastinal lymph nodes, vs. 8% after VAM(LA).
Current guidelines recommend invasive pre-resection staging in patients with cN1 disease and negative mediastinum on imaging. However, the choice between VAM or endosonography as first choice is left open (6). These recommendations are based on subgroup analysis of trials which included clinical stage I–III lung cancer patients (14). The vast majority of patients had cN2-disease, only a minority of the patients had cN1-disease with a normal mediastinum on imaging (7,8,15).
The double approach with endosonography first, followed by mediastinoscopy (if negative endosonography) is considered not cost-effective in the context of an occult N2 prevalence of less than 30% and sensitivity of endosonography of 38% to detect N2-disease (9). In the first study with 10 of 100 patients with positive mediastinum after endosonography, 90 should have been referred to mediastinoscopy by protocol. A NNT was calculated with 10 additional mediastinoscopies to find one extra patient with N2 disease. One can argue to omit an endosonography to evaluate mediastinal nodes and proceed directly to a surgical pre-resection staging by VAMLA in this patient group.
Whether invasive staging should be performed at all in patients with cN1 disease, is a different point of discussion that was not part of these studies. Some argue that invasive mediastinal staging might be unnecessary after negative mediastinum on PET-CT, as survival of unforeseen pN2 after resection is equivalent to cN2-disease (16) and survival after adjuvant therapy is similar to survival after neo-adjuvant therapy (17). Correct staging prior to the start of therapy is not only of paramount importance for comparative purposes, although it is responsible of an apparent better survival due to stage migration, it also leads to diverse surgical and non-surgical treatment strategies in individual patients, with potential individual benefits or avoidance of unnecessary treatments strategies. In these studies, at invasive staging, one third had multilevel N2 or N3 disease (9,10). Furthermore, more patients are able to have the full neo-adjuvant treatment preoperatively compared to postoperatively (18). With the advent of immunotherapy, and this therapy being investigated for resectable lung cancer, new theoretical advantages of preoperative therapy are being suggested (19). Invasive staging in patients with cN1 is indeed still recommended by current guidelines of ESTS and ESMO (6,20).
The combination of both studies does not equal a randomized controlled trial. While inclusion criteria and patient characteristics were similar, there was a time lapse and other centers that participated in both studies. After the results of the first prospective cohort study with low sensitivity of endosonography in this patient group, it seemed unethically to continue with a randomized trial. As VAM(LA) came after negative endosonography in the first study, a selection of patients occurred that potentially alters the measured sensitivity of VAM(LA) and the need for the second study became obvious.
The accrual rate of the second study on VAM(LA) was slower than originally anticipated, which resulted in slightly wider width of confidence interval than aimed for. Possibly, some potential patients were not included during the study period due to referral to endosonography for staging of mediastinal nodes, which was an exclusion criterion. Second, as the study was performed by institutions willing to participate in an prospective study on invasive staging, results can be different from the performance of the pre-resection staging in daily practice (21).
In 31% of patients in the second study, the mediastinoscopy was labelled as a VAMLA. In theory VAM and VAMLA are different procedures. In reality, procedures can often be labeled in-between as some stations are removed completely and others sampled within the same operation. While VAMLA goes beyond a pure diagnostic procedure and might be a first step in a complete lymphadenectomy, VAMLA should not be confused with transcervical extended mediastinal lymphadenectomy (TEMLA) which is performed through 5 to 8 cm cervical incision including elevation of the sternal manubrium and complete mediastinal lymphadenectomy except for stations 9 and most distal 4L (22). However, the false negative results were all found after a standard VAM, none after VAMLA. In two patients, the positive mediastinal nodes (both position 6) could by default not be reached by VAM or VAMLA. VAMLA performed therefore very well, with no false negative results and no complications. The numbers were too small to compare standard VAM with VAMLA, but in our opinion a pre-resection VAMLA can help to perform a complete mediastinal lymphadenectomy in these cN1 patients with clearly clinical significant risk of mediastinal nodal disease.
In conclusion, we prospectively analyzed the performance of pre-resection mediastinal staging with endosonography and VAM(LA) in a cohort of patients with cN1 (suspected) NSCLC. We confirmed that one in four eventually had N2 disease and found a sensitivity of 73% after VAM(LA). As endosonography alone had an unsatisfactory sensitivity to detect mediastinal disease, we argue to choose for VAMLA as preferred technique for pre-resection mediastinal nodal staging in patients with cN1 NSCLC.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors Marcin Zielinski and Qingdong Cao for the series “Mediastinoscopic Surgery” published in Mediastinum. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/med.2019.12.01). The series “Mediastinoscopic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Watanabe S, Asamura H, Suzuki K, et al. Problems in diagnosis and surgical management of clinical N1 non-small cell lung cancer. Ann Thorac Surg 2005;79:1682-5. [Crossref] [PubMed]
- Hishida T, Yoshida J, Nishimura M, et al. Problems in the current diagnostic standards of clinical N1 non-small cell lung cancer. Thorax 2008;63:526-31. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Ojha B, et al. Improving the inaccuracies of clinical staging of patients with NSCLC: a prospective trial. Ann Thorac Surg 2005;80:1207-13; discussion 1213-4. [Crossref] [PubMed]
- Kim D, Choi YS, Kim HK, et al. Heterogeneity of clinical n1 non-small cell lung cancer. Thorac Cardiovasc Surg 2014;62:103-8. [Crossref] [PubMed]
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive summary: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:7S-37S.
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA 2010;304:2245-52. [Crossref] [PubMed]
- Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 2011;142:1393-400.e1. [Crossref] [PubMed]
- Dooms C, Tournoy KG, Schuurbiers O, et al. Endosonography for mediastinal nodal staging of clinical N1 non-small cell lung cancer: a prospective multicenter study. Chest 2015;147:209-15. [Crossref] [PubMed]
- Decaluwé H, Dooms C, D'Journo XB, et al. Mediastinal staging by videomediastinoscopy in clinical N1 non-small cell lung cancer: a prospective multicentre study. Eur Respir J 2017; [Crossref] [PubMed]
- Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77.
- Lardinois D, De Leyn P, Van Schil P, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg 2006;30:787-92. [Crossref] [PubMed]
- Mizuno T, Arimura T, Kuroda H, et al. Histological type predicts mediastinal metastasis and surgical outcome in resected cN1 non-small cell lung cancer. Gen Thorac Cardiovasc Surg 2017;65:519-26. [Crossref] [PubMed]
- Tournoy KG, Keller SM, Annema JT. Mediastinal staging of lung cancer: novel concepts. Lancet Oncol 2012;13:e221-9. [Crossref] [PubMed]
- Kang HJ, Hwangbo B, Lee GK, et al. EBUS-centred versus EUS-centred mediastinal staging in lung cancer: a randomised controlled trial. Thorax 2014;69:261-8. [Crossref] [PubMed]
- Thomas DC, Arnold BN, Rosen JE, et al. The significance of upfront knowledge of N2 disease in non-small cell lung cancer. World J Surg 2018;42:161-71. [Crossref] [PubMed]
- Lim E, Harris G, Patel A, et al. Preoperative versus postoperative chemotherapy in patients with resectable non-small cell lung cancer: systematic review and indirect comparison meta-analysis of randomized trials. J Thorac Oncol 2009;4:1380-8. [Crossref] [PubMed]
- Felip E, Rosell R, Maestre JA, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol 2010;28:3138-45. [Crossref] [PubMed]
- Ghysen K, Vansteenkiste J. Immunotherapy in patients with early stage resectable nonsmall cell lung cancer. Curr Opin Oncol 2019;31:13-7. [Crossref] [PubMed]
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-21. [Crossref] [PubMed]
- Smulders SA, Smeenk FW, Janssen-Heijnen ML, et al. Surgical mediastinal staging in daily practice. Lung Cancer 2005;47:243-51. [Crossref] [PubMed]
- Zieliński M. Transcervical extended mediastinal lymphadenectomy: results of staging in two hundred fifty-six patients with non-small cell lung cancer. J Thorac Oncol 2007;2:370-2. [Crossref] [PubMed]
Cite this article as: Decaluwé H, Dooms C. Invasive mediastinal staging by endosonography or video-assisted mediastinoscopy in PET-CT clinical N1 non-small cell lung cancer. Mediastinum 2020;4:6.


